Pharmaceutical composition, its preparation method and usage
A composition and drug technology, applied to the pharmaceutical composition of angina pectoris and ischemic stroke and its preparation, the treatment of shock, and the field of coronary heart disease, which can solve the problems of difficulty in controlling the quality of injections, easy occurrence of adverse reactions, and unstable efficacy of preparations To achieve the effect of improving drug efficacy and stability, reducing drug dosage, and reliable curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0028] The proportioning screening test of experimental example 1 composition
[0029] (1) Comparison of effects on myocardial ischemia
[0030] 40 SD rats, male, weighing 250-350 g, were randomly divided into 4 groups: blank control group (equal volume normal saline), Shenmai group A (32:1, 20 mg / kg), group B (16:1, 20mg / kg), group C (4:1, 20mg / kg), group D (2:1, 20mg / kg). 6 rats / group were anesthetized by intraperitoneal injection of pentobarbital (30mg / Kg), fixed in supine position, endotracheal intubation, connected to ventilator, tidal volume 1ml, frequency 14 breaths / min, respiratory ratio 3:1. Adjust the physiological recorder, intubate the right common carotid artery, and connect a pressure transducer to measure arterial systolic blood pressure (SBP) and diastolic blood pressure (DBP). Connect limb leads to measure ECGII, and record the normal values of each parameter after stabilization. The left chest was shaved and disinfected. Along the left midline of the cla...
experiment example 2
[0045] Experimental Example 2 Pharmacodynamic Screening
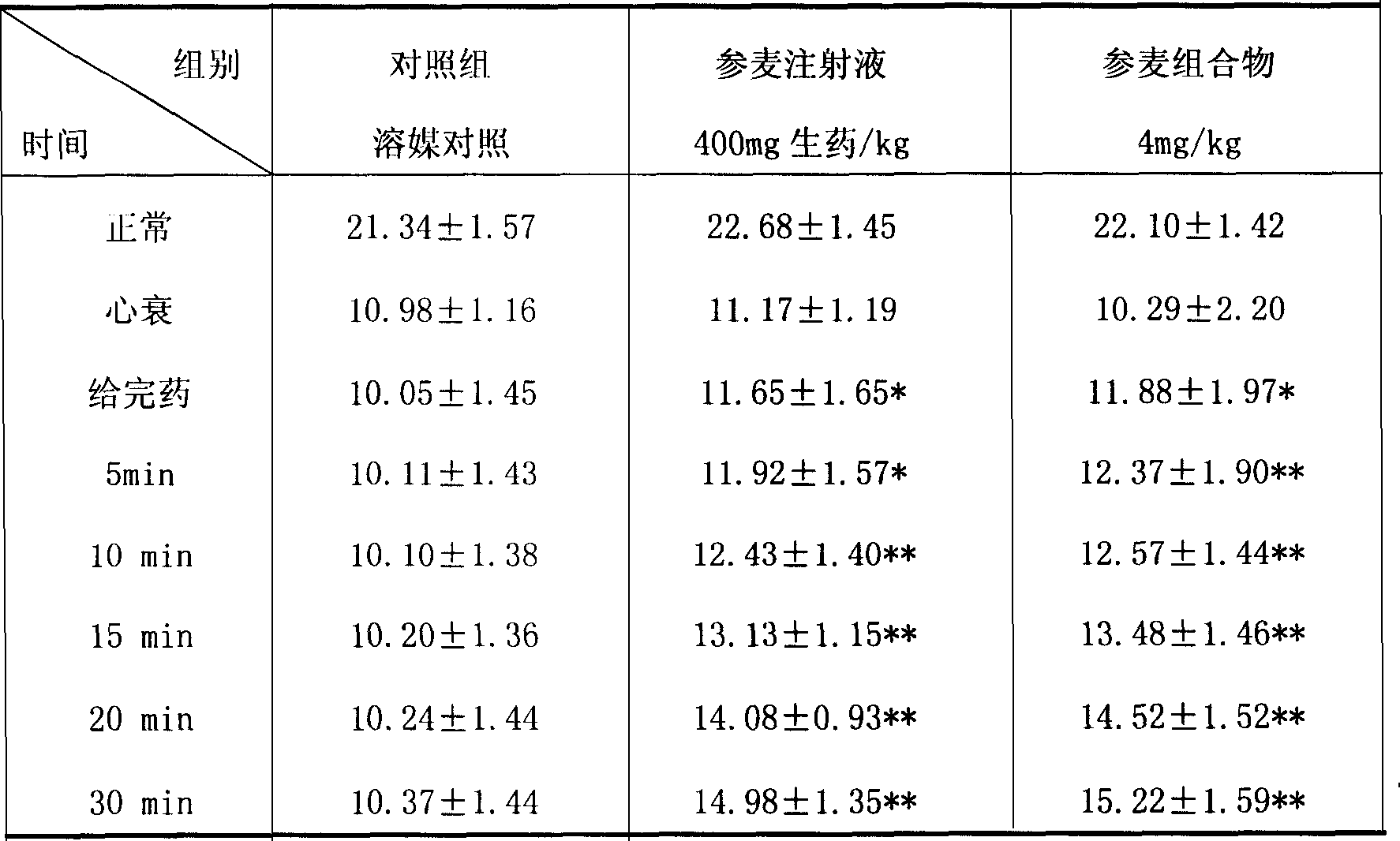
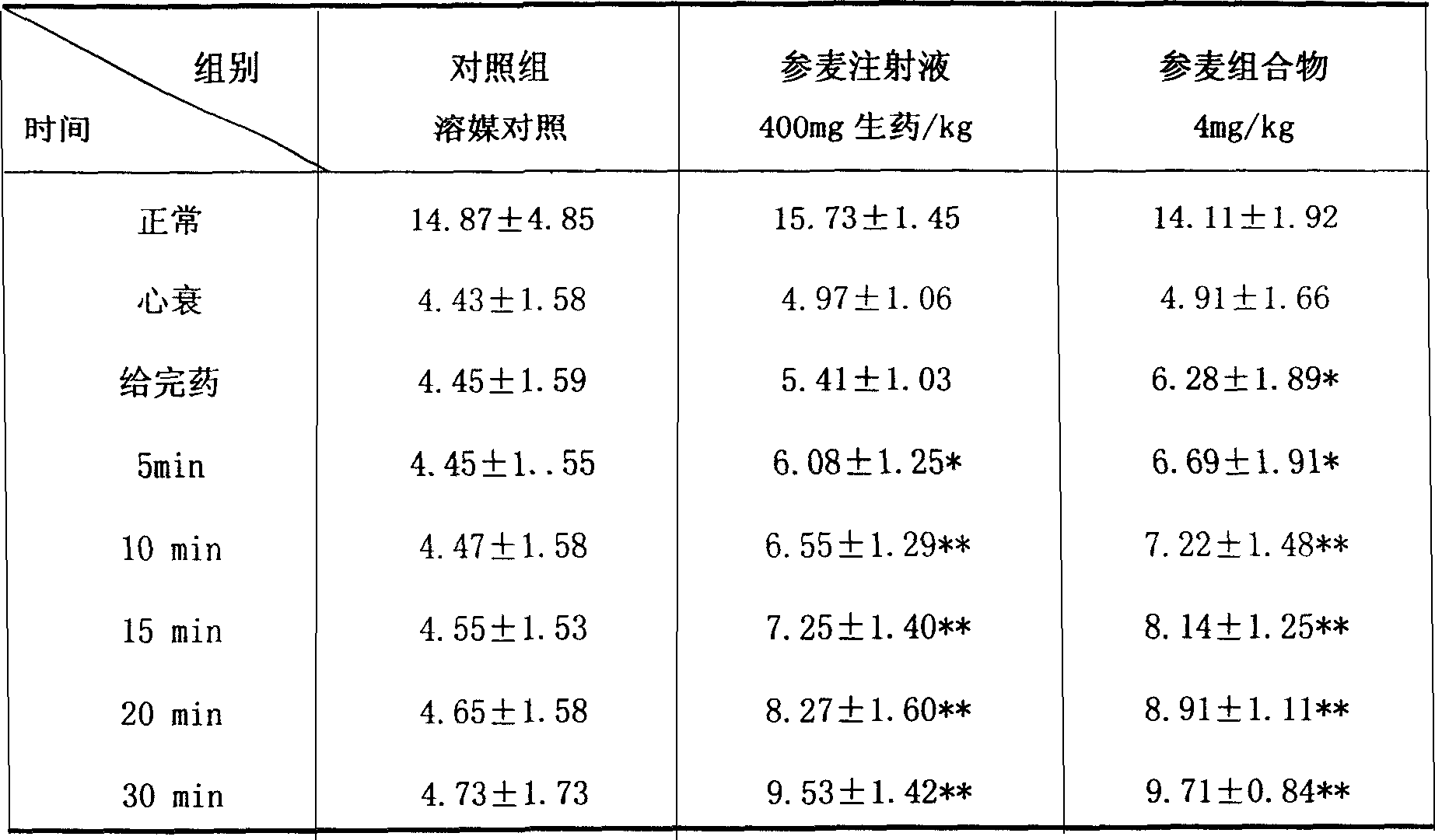
[0046] (1) Effects on cardiogenic shock caused by coronary artery ligation in anesthetized open-chest dogs
[0047] Thirty hybrid dogs were divided into 5 groups, 6 dogs in each group. The control group was given equal volume of vehicle control, the positive drug group was given Shenmai injection 3.2ml / kg (640mg crude drug / kg), and the experimental component was Shenmai composition (red ginseng extract: Ophiopogon japonicus extract 4:1) 4mg / kg dose group, all adopt slow infusion. Anesthetized with pentobarbital sodium 30mg / kg i.v., dorsal position fixed, neck skin incision, endotracheal intubation, connected to an electric ventilator, isolated right carotid artery, connected to an AP-601G amplifier, and measured blood pressure. After the experiment, the heart was removed, and the whole heart was weighed, and the roots of the great vessels and atrium were cut along the coronary sulcus, and the left ventricle was weigh...
Embodiment 1
[0066] Embodiment 1: Preparation of Shenmai Common Tablets
[0067] Take the red ginseng medicinal material, add 6 times the amount of crude drug with 50% ethanol to extract by heating and reflux for 0.5 hour, filter, and the filtrate is set aside, add 6 times the amount of 50% ethanol to the medicinal residue and extract 4 times, each time for 0.5 hour, filter, The four filtrates were combined, concentrated under reduced pressure, and the concentrated solution was placed at room temperature, slowly injected into a D101 macroporous adsorption resin column (the ratio of medicinal materials to dry resin was 3:1), and first eluted with 10 times the column volume of deionized water, and then Use 5 times of column volume for elution with 30% ethanol, discard 30% eluent, then elute with 70% ethanol, collect 60% of column volume for 6 times of column volume, recover ethanol and concentrate, and vacuum dry the concentrate , crushed to obtain red ginseng extract. Take Ophiopogon japon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com