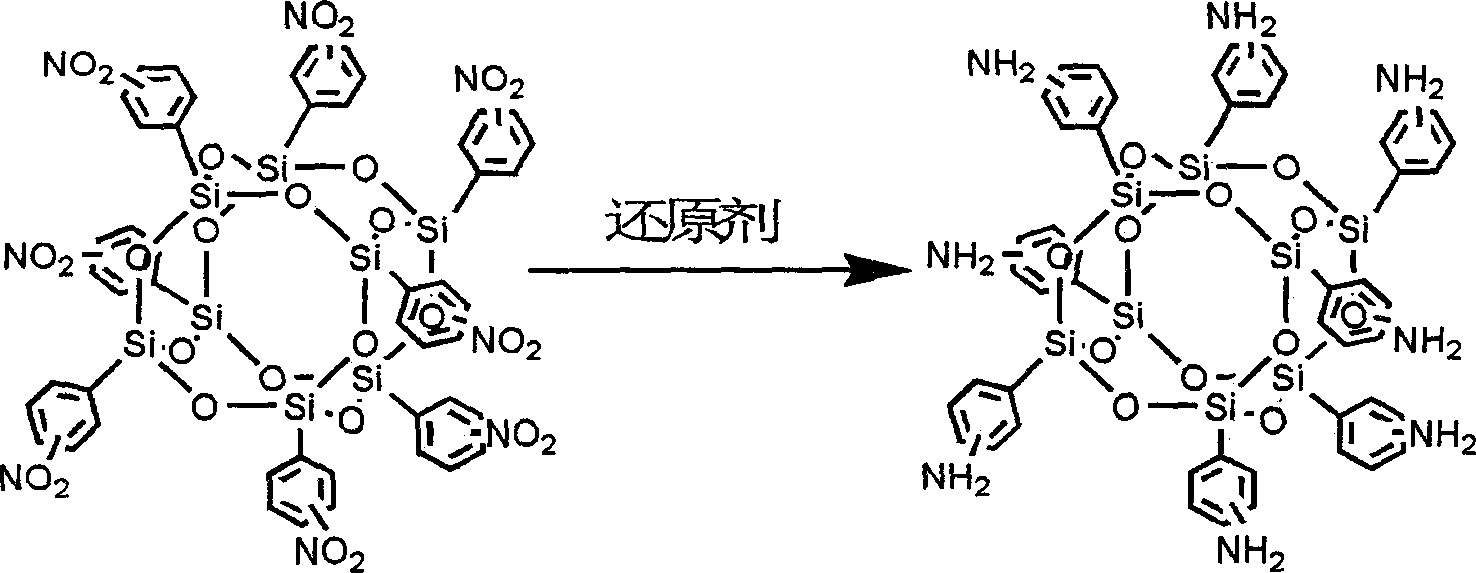
Process for preparing amino phenyl silsesquioxane
A technology of silsesquioxane and octaaminobenzene, which is applied in the field of nanomaterials to achieve the effect of improving heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Add 1.79 mmol (2.5 g) of octanitrophenyl cage silsesquioxane (hereinafter referred to as ONPS), 50 mL of tetrahydrofuran, and 2 g of Fe / C supported catalyst into a 500 mL three-necked flask, stir and mix, and heat up to 60° C.; add hydrazine hydrate (80%), the weight ratio of hydrazine hydrate to ONPS is 3:1, react for another 4 hours, and stop the reaction. Cool down to room temperature, filter, add ethyl acetate for extraction, precipitate in petroleum ether, separate and dry to obtain a white precipitate, namely octaaminophenyl cage silsesquioxane, with a yield of 82% and a reduction efficiency of 100%.
Embodiment 2
[0022] Embodiment 2: prepare octapolyaminophenyl cage type silsesquioxane with the method of embodiment 1, operation step is identical with example 1, and difference is that the weight ratio of hydrazine hydrate and ONPS is 2: 1, and solvent is 1 , 4-dioxane, and the reaction temperature is 100° C. to finally obtain octaaminophenyl cage silsesquioxane with a yield of 77% and a reduction efficiency of 100%.
Embodiment 3
[0023] Embodiment 3: Prepare octaaminophenyl cage type silsesquioxane with the method of embodiment 1, operation procedure is identical with example 1, difference is octanitrophenyl cage type silsesquioxane, solvent, Fe The weight ratio of the / C catalyst three is 10: 200: 10, the weight ratio of hydrazine hydrate and ONPS is 8: 1, and the temperature of reaction is 50 ℃, finally obtains octaaminophenyl cage silsesquioxane, productive rate 71 %, the reduction efficiency is 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com