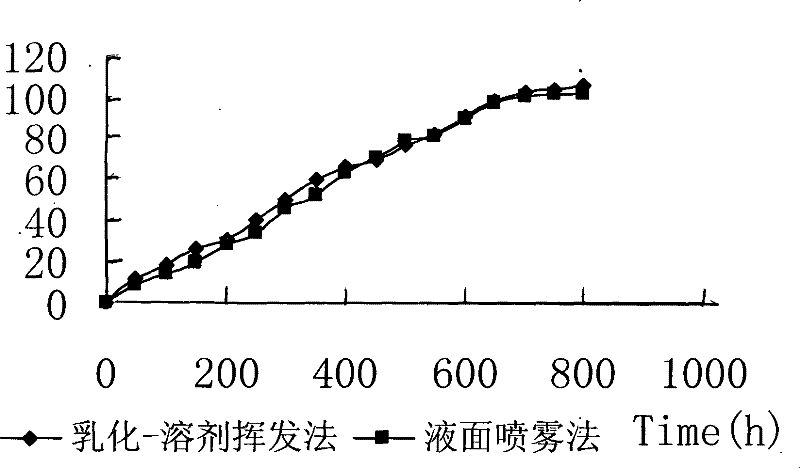
Preparation of new drug delivery system based on liquid surface spray method
A technology of delivery system and spray method, which is applied in the application of liquid surface spray method in drug delivery system, and the application field of preparing a new type of drug delivery system based on liquid surface spray method in industrialized large-scale production, which can solve the problem of unseen liquid In order to achieve the effect of similar in vitro release properties, good reproducibility, and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The preparation of embodiment 1 vinpocetine lactic acid-glycolic acid copolymer (PLGA) microsphere
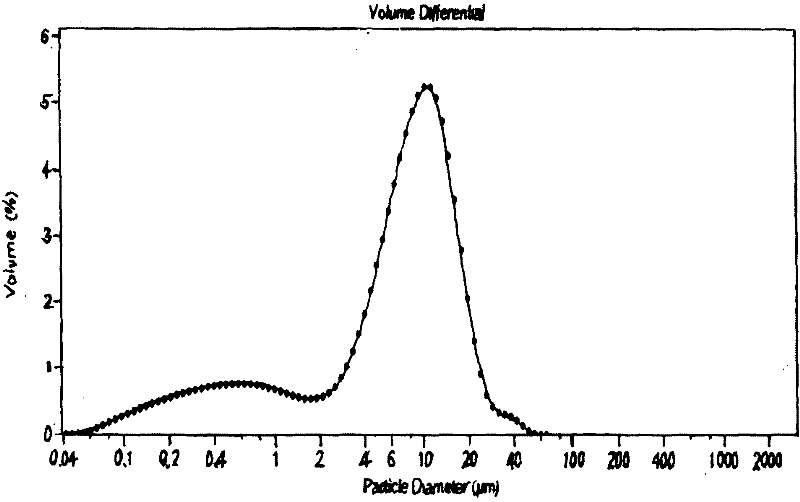
[0016] At room temperature, accurately weigh 50 mg of the drug Vinpocetine and 200 mg of the polymer PLGA dissolved in 10 ml of dichloromethane as the atomization phase, and measure 50 ml of 1% PVA aqueous solution as the aqueous phase receiving solution. Spray the atomized phase into the receiving solution at a flow rate of 2.0ml / min and a pressure of 0.15MPa through a spray device, stir and volatilize at room temperature, centrifuge at 3000r / min, and wash to collect. The particle size distribution of the microspheres was determined to be narrow, and the encapsulation efficiency was 83.3%.
Embodiment 2
[0017] The preparation of embodiment 2 metformin hydrochloride gelatin microspheres
[0018] Weigh 180mg of metformin hydrochloride and add it to 10ml of 20% gelatin aqueous solution kept in a 50°C water bath as an atomization phase; at the same time, add an appropriate amount of glutaraldehyde to 50ml of a liquid containing 1% Span-80 kept in a 50°C water bath In paraffin, as the receiving liquid; spray the atomized phase into the receiving liquid at a flow rate of 15.0ml / min and a pressure of 0.35MPa through a spray device, and at the same time stir at room temperature, after cross-linking, add 20ml of isopropanol, fully dehydrated, pump rate, washed with isopropanol, anhydrous ether, and anhydrous ethanol, respectively, and dried in vacuum. Compared with the traditional method, the particle size distribution of microspheres is narrow, and the encapsulation efficiency and drug dosage are both improved.
Embodiment 3
[0019] The preparation of embodiment 3 intravenous injection fat emulsion (blank emulsion)
[0020]
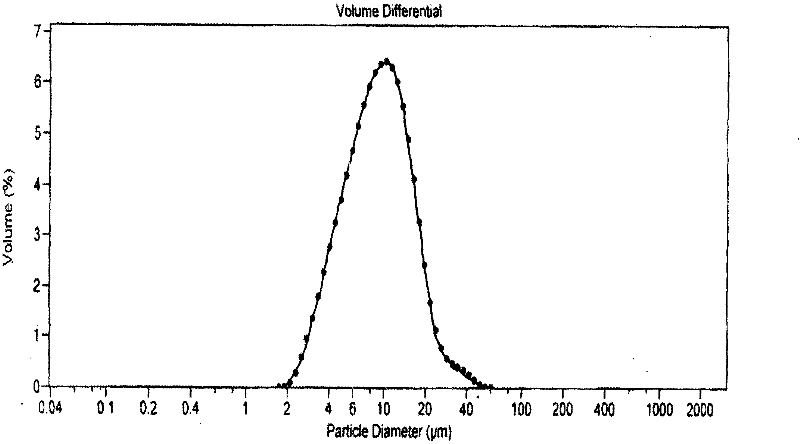
[0021] Operation: Add phospholipid (Lecithin170) to soybean oil kept warm in a water bath at 70°C to form an oil phase (receiving solution). Add glycerin to an appropriate amount of water and keep warm in a water bath at 70°C to form a water phase (atomized phase); Spray the atomized phase into the receiving liquid at a flow rate of 4ml / min and a pressure of 0.2MPa to form colostrum, and then disperse it by ultrasonic to form a stable emulsion. Compared with the traditional method, the prepared emulsion is more stable.
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com