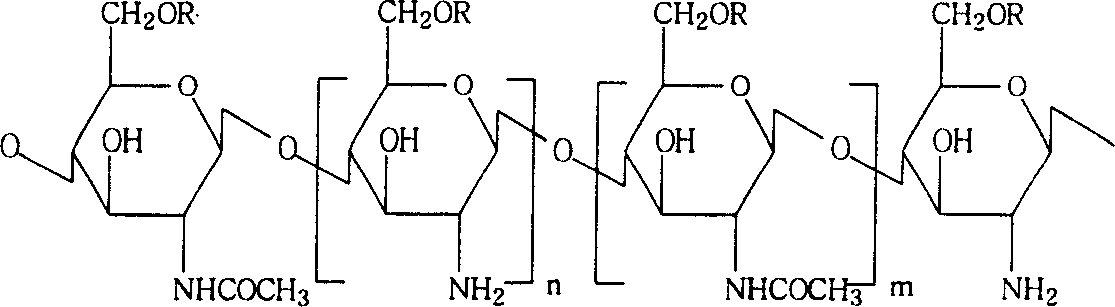
Kidney-targeted medicine vector and the formed prodrug, preparation method and uses
A kidney-targeted and drug-based technology, applied in the field of new kidney-targeted prodrugs and preparations, can solve problems such as preparation methods and applications that have not been proposed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of low molecular weight water-soluble chitosan
[0060] Dissolve 3g of chitosan in 150ml of 0.45% (w / v) hydrochloric acid aqueous solution, after completely dissolving, filter, add 1.88ml of hydrogen peroxide, and stir and react at 40-70°C for 2 hours. Adjust the pH value to 13 with sodium hydroxide, precipitate out, centrifuge at 4000 r / c to obtain a low molecular weight chitosan precipitate, wash with water until neutral, and then vacuum dry.
[0061] Take 1g of the dry product and dissolve it in 170ml of 3% acetic acid solution. After completely dissolving, add 6ml of acetic anhydride, stir and react at room temperature for 4 hours, adjust the pH value to 13 with sodium hydroxide, add 500ml of acetone, and centrifuge to obtain a precipitate , the precipitate was treated with 1 mol / L potassium hydroxide in methanol for 5 hours, washed with methanol to neutrality, dissolved in water, and further purified by gel permeation chromatography (GPC). After freeze...
Embodiment 2
[0063] Preparation and in vivo distribution of fluorescently labeled chitosan
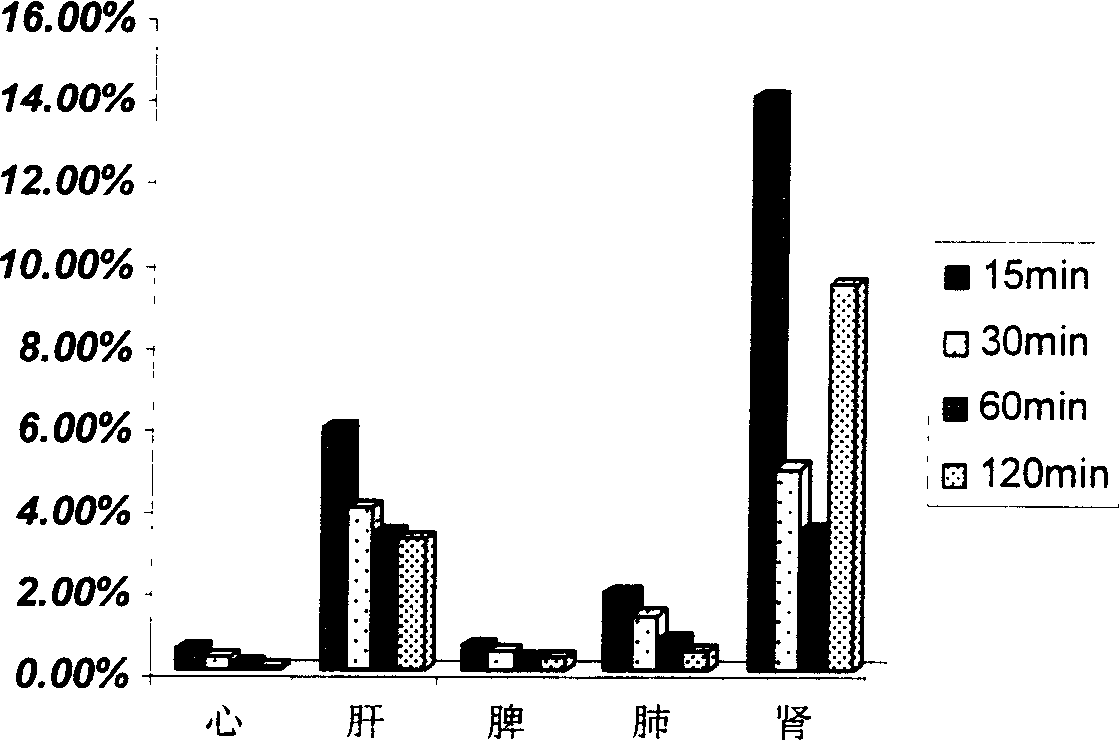
[0064] Accurately weigh 300mg of chitosan (Mw=20000, deacetylation degree is about 50%, water-soluble) and dissolve in 10ml of 1mol / L HCl solution, then adjust the pH value to 6.9 with NaOH. Add 21 mg FITC (fluorescein isothiocyanate), mix well, react at room temperature for 24 hours, and dialyze. The dialyzed sample was freeze-dried (chitosan itself is a good scaffold base, so there is no need to add other scaffold bases). The fluorescein content was determined to be 6.56% (w / w). Take 20 Kunming mice, male, weighing between 25-30 grams, each group of 5 mice, a total of 4 groups. Calculated according to the injection dose of 40mg / kg, inject FITC-chitosan into the tail vein, kill the mice at 15min, 30min, 60min, and 120min respectively, take the heart, liver, spleen, lung, and kidney, weigh them, and prepare a tissue homogenate. After the protein was precipitated, the content was measured with a ...
Embodiment 3
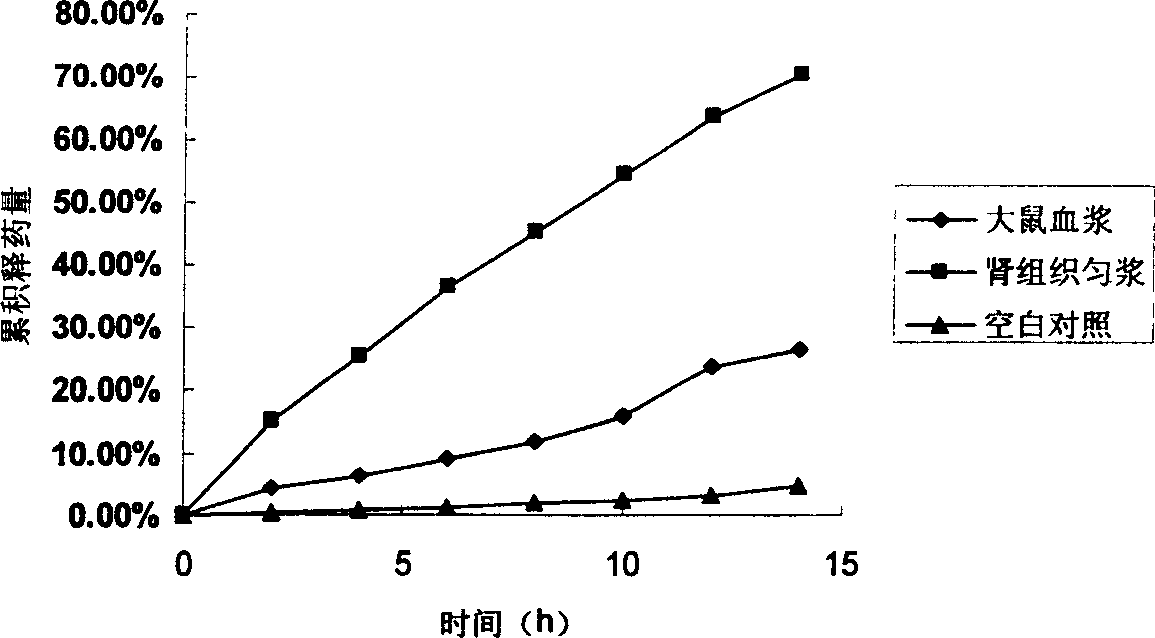
[0066]Preparation of prednisolone succinate-chitosan prodrug
[0067] Precisely weighed 115mg of prednisolone succinate monoester, 28.8mg of N-hydroxysuccinimide, dissolved in 20ml ethanol; accurately weighed 100mg of chitosan (Mw=10000 Dalton, deacetylation degree is 50% left and right, water-soluble) dissolved in 30ml of distilled water, after complete dissolution, the ethanol mixture was slowly added dropwise to the water phase under stirring conditions, and finally 120mg of 1-(3-dimethylaminopropyl)-3-ethylcarbadiene was added Imine hydrochloride was used as a condensation agent, reacted overnight at 4°C, precipitated the conjugate with 150ml of acetone, washed the precipitate several times, and dried to obtain the prodrug. Sealed and stored at low temperature.
[0068] Accurately weighed 115mg of prednisolone succinate, 28.8mg of N-hydroxysuccinimide, dissolved in 20ml of ethanol; accurately weighed 100mg of chitosan (Mw=20000 Daltons, water insoluble), added 10ml After...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mw | aaaaa | aaaaa |
| Mw | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com