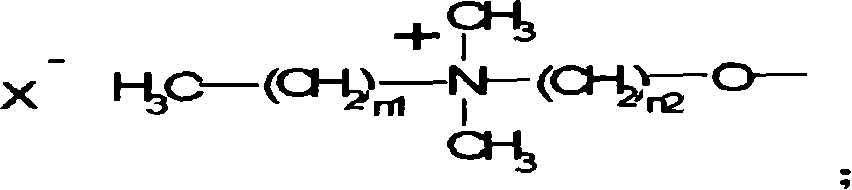
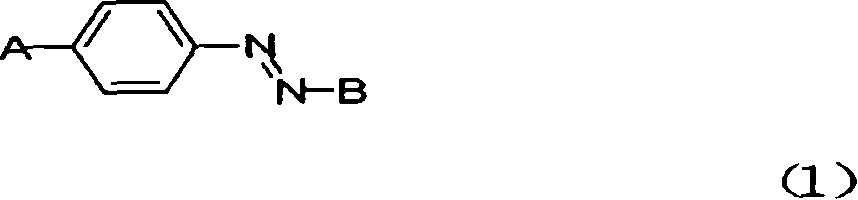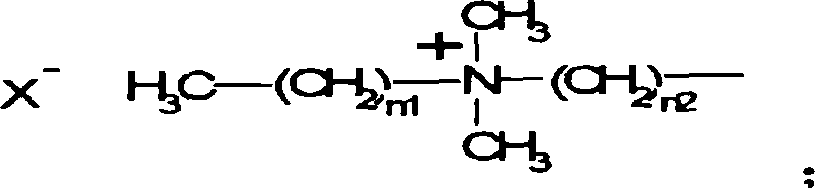Antibiotic azo dye comprising quaternary ammonium salt group and its preparation and uses
A technology of quaternary ammonium salt groups and azo dyes, applied in the direction of azo dyes, monoazo dyes, organic dyes, etc., can solve the problems of large energy consumption, waste water environmental pollution, etc., achieve slow dyeing speed, and simple synthesis method Easy to use and good dyeing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] preparation.
[0038] Synthetic route (1):
[0039]
[0040] Synthesis of Intermediate 1: Add 10g of 4-nitrophenol, 6g of sodium hydroxide, and 150ml of water into a four-neck flask, heat to dissolve, add 60g of dibromoethane, 0.25g of tetrabutylammonium bromide, and react at 90°C 12 hours. Remove water and dibromoethane under reduced pressure, dissolve the residue with hot ethanol, remove insoluble salt, cool, and the product is precipitated from ethanol to obtain 15 g of product; yield: 79%. The melting point of the product is 64°C, which is the target product;
[0041] Synthesis of Intermediate 2: Add 20 g of NN-dimethyldodecylamine to 8 g of Intermediate 1, and react at 60° C. for 7 hours; wash the crude product with cyclohexane to obtain 14.25 g of the product, yield: 95%;
[0042] Synthesis of intermediate 3: 7g of intermediate 2 was dissolved in 50ml of ethanol, and a mixture of 14g of tin protochloride and 19g of concentrated hydrochloric acid with a wei...
Embodiment 2
[0049] As shown in the synthetic route (1), the synthesis of intermediates 1, 2 and 3 is shown in Example 1;
[0050] Synthesis of Dye 5: Add 0.8g of Intermediate 3, 1.6ml of concentrated hydrochloric acid, add dropwise an aqueous solution containing 0.131g of sodium nitrite in an ice bath (0-2°C), stir and react for 2 hours to obtain a diazonium solution. 0.35 g of 1-phenyl-3-methyl-5-pyrazolone was dissolved in 20 ml of sodium carbonate aqueous solution to obtain a coupling solution. Add the coupling solution to the diazo solution at one time, control the pH value of the system from slightly acidic to neutral, and react at 5~15°C for 5 hours. Salt out with sodium chloride, filter out the precipitate, dry, dissolve the precipitate with absolute ethanol, filter off the insoluble salt, and remove the ethanol under reduced pressure to obtain 0.977g of dye; yield: 85%.
[0051] The total yield of dye 5 is 47.8%;
[0052] IR (KBr, cm -1 ): 3440(-NH-); 2920, 2850(CH 3 (CH 2 ...
Embodiment 3
[0056] the preparation of
[0057] The synthetic route of the dye is as follows:
[0058]
[0059] Synthesis of Intermediate 6: Add 5 g of p-nitrobenzyl bromide, add 15 g of N,N-dimethyldodecylamine, and react at 60°C for 5 hours; wash the crude product with cyclohexane to obtain 9 g of the product, yield: 92.1%;
[0060] Synthesis of Intermediate 7: Dissolve 4g of Intermediate 6 in 30ml of ethanol, add dropwise 8g of stannous chloride and 10g of a mixture of concentrated hydrochloric acid with a weight concentration of 36% under stirring, and react at 50°C for 8 hours. After the reaction, cool down to room temperature, add concentrated alkali, adjust the pH value to be alkaline, filter, and decompress the filtrate to obtain the crude product, add absolute ethanol to dissolve, filter out the insoluble salt, and remove the ethanol under reduced pressure to obtain 2.98g of product , Yield: 80%;
[0061] Synthesis of dye 8: 1g of intermediate 7, 2.1ml of concentrated hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com