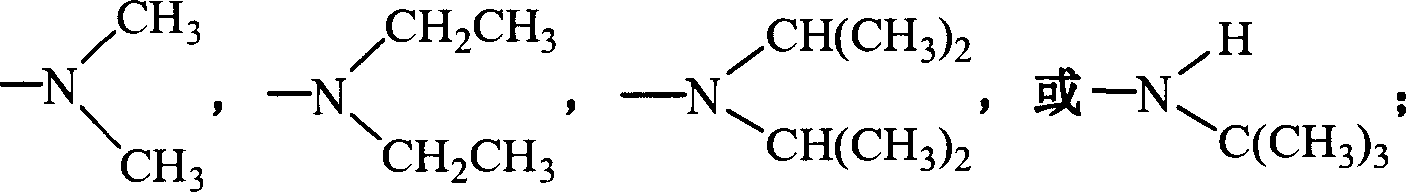
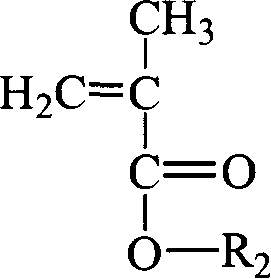
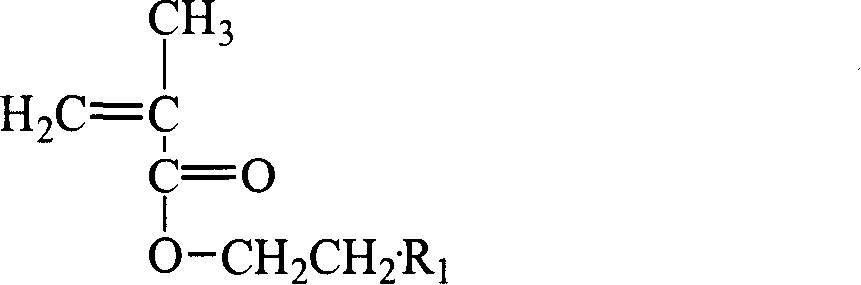Fluorine-containing superbranching-grafting block polymer and its preparation
A technology of block polymers and hyperbranched polymers, applied in the field of copolymers, can solve the problems of harsh living anionic polymerization conditions, difficult removal, difficult industrial control and production, etc., to improve the dual response performance of temperature and pH, good affinity Water-based, the effect of reducing surface energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment one: HPE-g-DMAEMA 15 -b-OFPMA 5 and its preparation
[0060] (1) Preparation of KH: Put the stirring rotor into a dry reaction bottle in advance, and plug it tightly with a reversible rubber stopper. Then use a needle, a latex tube, and connect it to a vacuum pump, and fill it with high-purity argon gas while evacuating it, and repeat the operation three times. After moving a certain amount of KH into the reaction bottle, inject 5 mL of dry THF with a dry syringe, stir and wash, and suck out the THF containing mineral oil with a syringe after resting, repeat this three times, and finally dry the residual THF solution with high-purity argon. Accurately weigh the amount of KH (0.05-0.15 g, about 1.25-3.75 mmol) in the reaction vial by subtraction method.
[0061] (2) Preparation of initiator: inject 20 mL of THF into the polymerization bottle. Place the reaction vial in an ice-water bath with magnetic stirring. Prepare the polymerization bottle that agitat...
Embodiment 2
[0063] Embodiment two: HPE-g-DMAEMA 15 -b-OFPMA 10 and its preparation
[0064] (1) Preparation of KH: same as Example 1.
[0065] (2) Preparation of initiator: same as Example 1.
[0066] (3) Polymerization: According to the length of the required graft chain (first monomer polymer chain) and block chain (second monomer polymer chain), control the oxygen anion in the first monomer and macroinitiator The molar ratio (measured in the molar concentration corresponding to the initiator) is 15: 1, and the first monomer methacrylate-2-(dimethylamino)ethyl (dimethylamino) ethyl ester ( DMAEMA), reacted for 0.5~1.5h; then control the molar ratio of the second monomer and the initiator oxyanion to be 10:1, inject the second monomer methacrylic acid- (2,2,3,3,4,4,5,5-octafluoro)pentyl ester (OFPMA), react for 0.5-1.5h, and finally terminate the reaction with dry methanol. The reacted polymer is subjected to rotary evaporation at 60-70°C to remove the solvent, followed by precipitati...
Embodiment 3
[0067] Embodiment three: HPE-g-DMAEMA 30 -b-OFPMA 10 and its preparation
[0068] (1) Preparation of KH: same as Example 1.
[0069] (2) Preparation of initiator: same as Example 1.
[0070] (3) Polymerization: According to the length of the required graft chain (first monomer polymer chain) and block chain (second monomer polymer chain), control the oxygen anion in the first monomer and macroinitiator The molar ratio (measured with the molar concentration corresponding to the initiator) is 30: 1, and the first monomer methacrylate-2-(dimethylamino) ethyl ester (dimethylamino) ethyl ester ( DMAEMA), reacted for 0.5~1.5h; then control the molar ratio of the second monomer and the initiator oxyanion to be 10:1, inject the second monomer methacrylic acid- (2,2,3,3,4,4,5,5-octafluoro)pentyl ester (OFPMA), react for 0.5-1.5h, and finally terminate the reaction with dry methanol. The reacted polymer is subjected to rotary evaporation at 60-70°C to remove the solvent, followed b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com