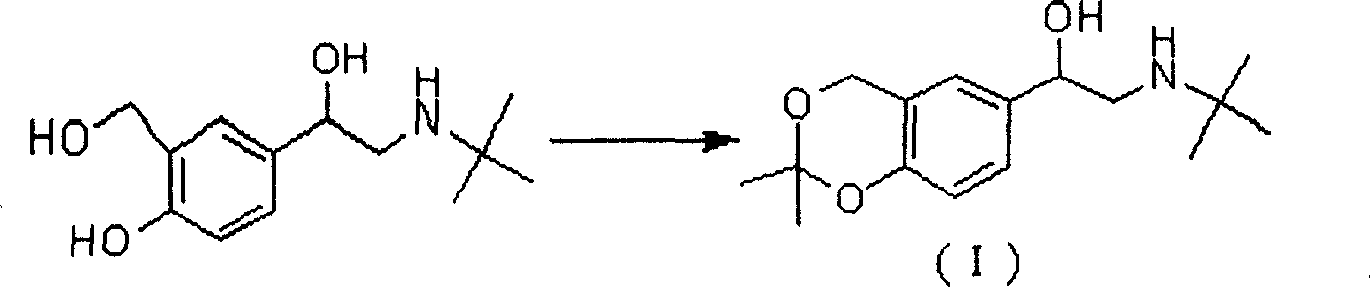Synthesis method of levorotatory albuterol hydrochloride
A technology for levsalbutamol and salbutamol, which is applied in the field of synthesis of levalbuterol hydrochloride, can solve the problems of difficulty in extracting salbutamol, difficult industrialized production, low optical purity and the like, and achieves the effects of cheap raw materials, stable finished products, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of 2-(N-tert-butylamino)-1-(2,2-dimethyl-4H-benzo[1,3]dioxo-6-yl)ethanol (I):
[0031] Add racemic albuterol (38g, 0.16mol) into acetone (400ml, 5.4mol) under nitrogen protection, cool with ice salt, add boron trifluoride ether (43ml, 0.35mol) dropwise, after the addition is complete, react at 0°C for 1h, and The mixture was poured into 390 g of 10% sodium hydroxide solution with a weight concentration of 0° C., stirred, the excess acetone was evaporated to dryness under reduced pressure, extracted with ethyl acetate (150 ml×3), the combined organic phases were washed with saturated brine , dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain 38 g of light yellow solid (I), yield: 85%, which was directly used in the next reaction without purification.
Embodiment 2
[0033] Preparation of 2-(N-tert-butylamino)-1-(2,2-dimethyl-4H-benzo[1,3]dioxo-6-yl)ethanol (I):
[0034] Add racemic albuterol (38g, 0.16mol) into acetone (800ml, 11mol) under nitrogen protection, cool with ice salt, add boron trifluoride diethyl ether (59ml, 0.48mol) dropwise, after the addition is complete, react at -10°C for 2h. The mixture was poured into 535 g of 10% sodium hydroxide solution with a weight concentration of 0° C., stirred, the excess acetone was evaporated to dryness under reduced pressure, extracted with ethyl acetate (150ml×3), the combined organic phases were washed with saturated brine , dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain 42 g of light yellow solid (I), yield: 94%, which was directly used in the next reaction without purification.
Embodiment 3
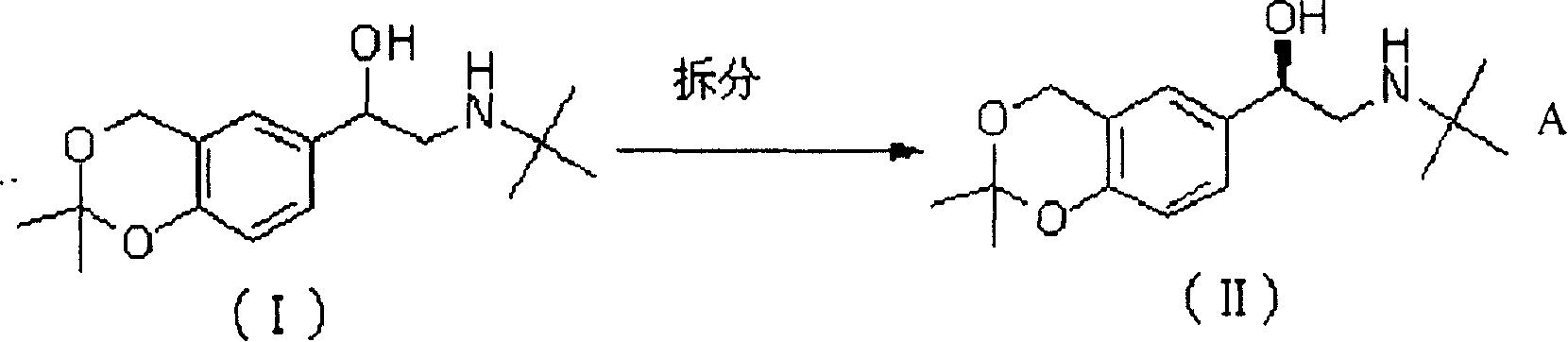
[0036] (1) R-2-(N-tert-butylamino)-1-(2,2-dimethyl-4H-benzo[1,3]dioxo-6-yl)ethanol. The preparation of D-(+)-dibenzoyl tartrate (II):
[0037] Methanol (135ml), was added to the mixture of compound (I) (17g, 0.061mol) and D-(+)-dibenzoyl tartaric acid (11.8g, 0.033mol), heated to reflux for 30min, gradually cooled to room temperature within 30min , stirred at 5°C for 1h, filtered, washed the solid with ethyl acetate (20ml), and dried to give 9.2g of white solid (II), yield: 65% (for single isomer), 88.8% ee
[0038] (2) R-2-(N-tert-butylamino)-1-(2,2-dimethyl-4H-benzo[1,3]dioxo-6-yl)ethanol. Recrystallization of D-(+)-dibenzoyl tartrate (II)
[0039] The solid (II) (9.2g, 0.02mol) obtained in Example 3.1 was added to methanol (135ml), heated to reflux for 2h, gradually cooled to room temperature, stirred at 2°C for 1h, filtered, and the solid was washed with ethyl acetate (20ml) , dried to obtain a white solid (II), repeat the above operation twice to obtain a white solid (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com