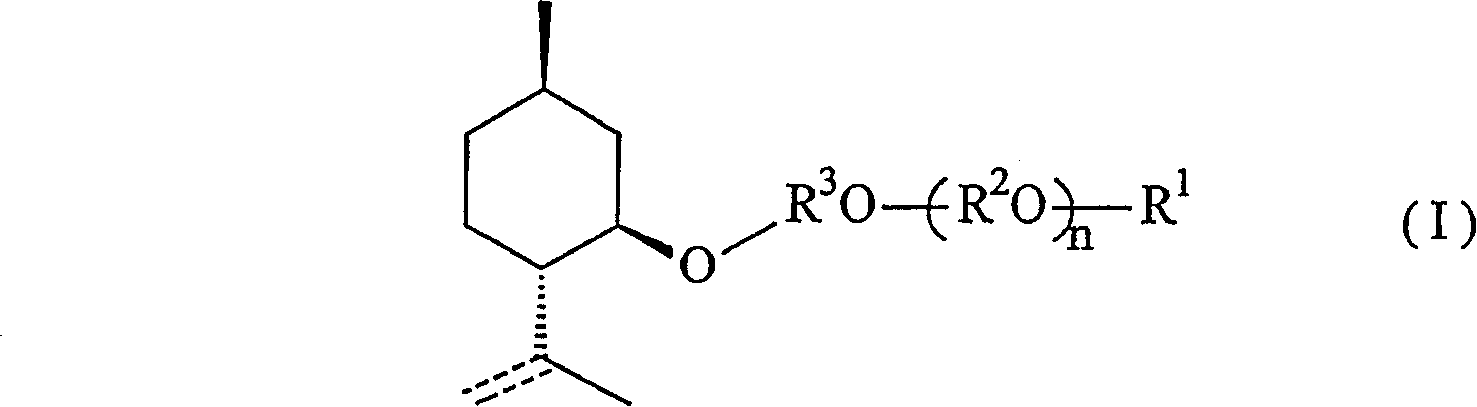
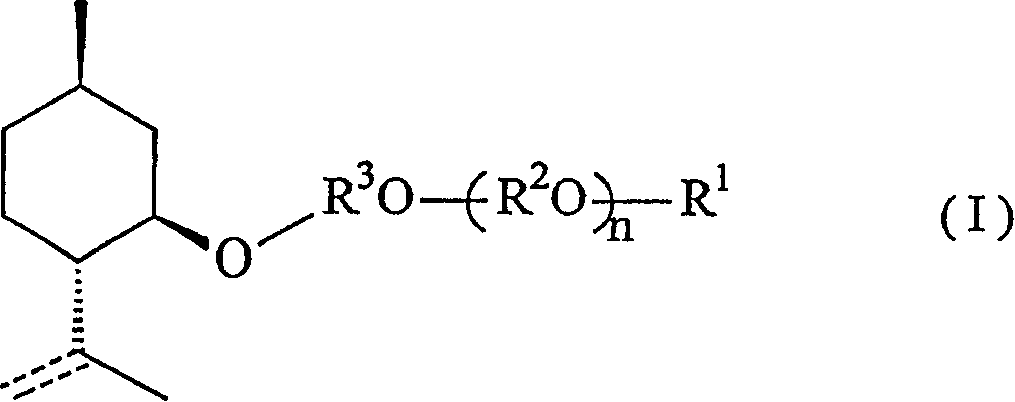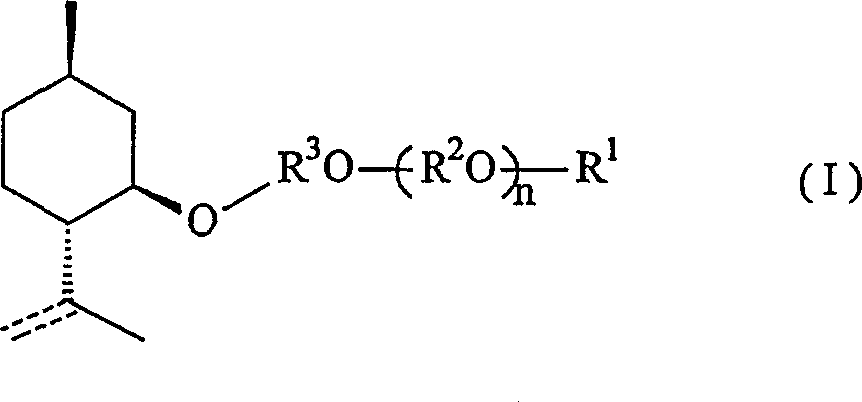Menthol derivative and cooling agent composition comprising the same
A technology of menthol derivatives and compositions, applied in the fields of beverages or foods, bathing agents or medicines, cosmetics, cosmetic products, and sensory stimulant compositions, which can solve the problem of insufficient and unsatisfactory retention of cooling effects, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0065] Product Synthesis Examples and Examples were measured with the following equipment.
[0066] NMR spectroscopy:
[0067] 1 H-NMR: AM-400 (400 MHz), produced by BRUKER Inc.
[0068] External standard: tetramethylsilane
[0069] Gas Chromatography (GC):
[0070] HP 6890, produced by HEWLETT PACKARD Inc.
[0071] Chromatographic column: NEUTRABOND-1, produced by GL Science Inc. (inner diameter×height=0.25mm×30m).
[0072] Mass spectrometry (MS):
[0073] M-80 mass spectrometer, manufactured by Hitachi Ltd. (ionization voltage 20 eV).
Embodiment 1
[0075] [Synthesis of 2-(2-1-menthoxyethyl)ethanol]
[0076] (1) Synthesis of 1-menthoxyethanol
[0077]
[0078] First, add 100.0g (0.64mol) of 1-menthol and 42.7g (0.32mol) of anhydrous aluminum chloride into the reactor, replace with nitrogen, then add 500ml of toluene and stir the mixture. The mixture was brought to 5°C. And when the temperature of the solution was kept at 5-10° C., 57 g of ethylene oxide was further added. After the addition was complete, the mixture was stirred at room temperature for an additional 1 hour to complete the reaction. Next, the reaction solution was cooled again, and washed with 150 ml of a 10% aqueous hydrochloric acid solution while maintaining the temperature at 20°C or lower. The reaction solution was washed with 50 ml of saturated aqueous sodium bicarbonate solution, then with 100 ml of saturated brine, and the water was removed with sodium sulfate, and the solvent was recovered under reduced pressure to obtain 123.1 g of crude 1-m...
Embodiment 2
[0088] [Preparation of 2-(2-1-menthoxyethyl)ethanol with NaH]
[0089] The 100ml capacity four-necked flask of Dimroth condenser and thermometer is installed, the 1-menthol of 78.0g (0.5mol) and the NaH (60%) of 20g (0.5mol) are housed, after replacing with nitrogen, add 160ml toluene. The mixture was heated to reflux and stirred for 2 hours. The reaction solution, from which no hydrogen evolution was observed, was cooled to 60° C., at which temperature 44 g (1.0 mol) of ethylene oxide was added within 1 hour. After reflux and stirring for 2 hours, the reaction solution was cooled again and washed with 100 ml of 1% aqueous hydrochloric acid while maintaining the temperature at 10°C or lower. Then, the reaction solution was washed with 50 ml of saturated aqueous sodium bicarbonate, then with 100 ml of saturated brine, water was removed with sodium sulfate, and the solvent was recovered under reduced pressure, then distilled, separated, and reclaimed unreacted 1-menthol and 2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com