RE perovskite type catalyst for oxidizing NO
A nitric oxide and catalyst technology, which is applied in the field of rare earth perovskite catalysts, can solve the problems of limiting the application of nitric oxide oxidation catalysts, high cost and the like, and achieves the effects of abundant raw materials, convenient operation and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
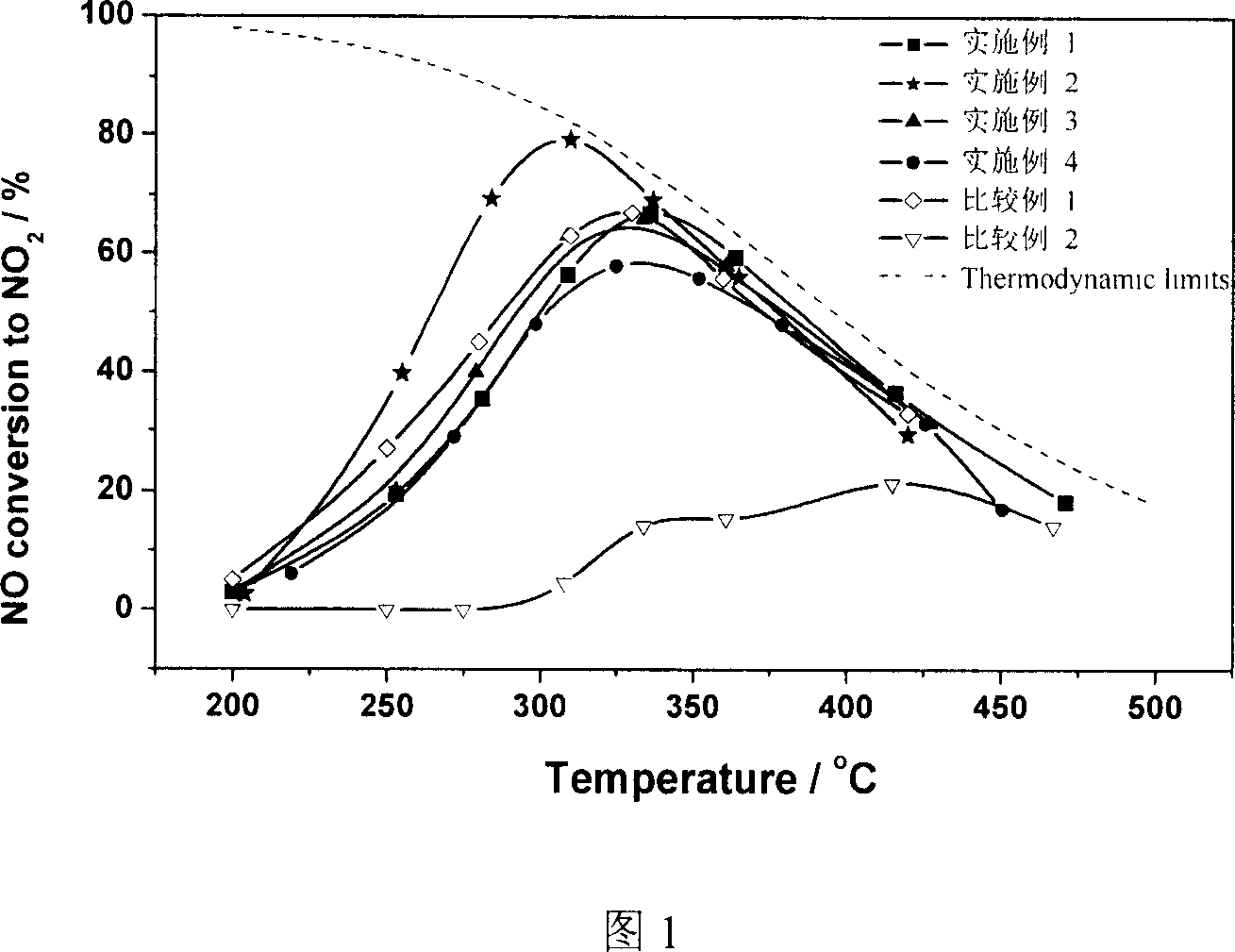
[0016] Lanthanum nitrate [La(NO 3 ) 3 ·6H 2 O] 43.3 grams (0.100 moles), cobalt nitrate [Co(NO 3 ) 2 ·6H 2 O] 29.1 g (0.100 mol) was dissolved in 200 ml of deionized water and stirred thoroughly. Citric acid [C 6 h 8 o 7 ·H 2 O] 42.0 g (0.200 mol) was dissolved in 100 ml of deionized water solution and stirred thoroughly, then slowly poured into the metal salt solution, stirred for half an hour, poured into a pear-shaped glass flask, and rotated under reduced pressure at 60-80°C Evaporation, the water was evaporated to dryness to obtain a viscous complex. The precursor was moved to an evaporating dish and dried overnight at 120°C to obtain an expanded dry powder with strong hygroscopicity, which was ground and calcined at 700°C for 2 hours to obtain a black powder sample.
[0017] According to the powder X-ray diffraction analysis results, the powder is made of LaCoO 3 A single crystal composed of a perovskite-type composite oxide. Its specific surface area is 10 m...
Embodiment 2
[0019] Lanthanum nitrate [La(NO 3 ) 3 ·6H 2 O] 34.6 grams (0.080 moles), cerium nitrate [Ce(NO 3 ) 3 ·6H 2 O] 8.7 grams (0.020 moles), cobalt nitrate [Co(NO 3 ) 2 ·6H 2 O] 29.1 g (0.100 mol) was dissolved in 200 ml of deionized water and stirred thoroughly. Others were operated in the same manner as in Example 1 to obtain a black powder sample.
[0020] According to the powder X-ray diffraction analysis results, the powder is made of La 0.9 Ce 0.1 CoO 3 Composite oxides with a perovskite structure and a small amount of CeO 2 and CoO x composed mixture. The specific surface area is 13 m2 / g.
Embodiment 3
[0022] Lanthanum nitrate [La(NO 3 ) 3 ·6H 2 O] 26.0 grams (0.060 moles), cerium nitrate [Ce(NO 3 ) 3 ·6H 2 O] 17.4 grams (0.040 moles), cobalt nitrate [Co(NO 3 ) 2 ·6H 2 O] 14.6 grams (0.050 moles), nickel nitrate [Ni(NO 3 ) 2 ·6H 2 O] 14.5 g (0.050 mol) was dissolved in 200 ml of deionized water and stirred thoroughly. Others were operated in the same manner as in Example 1 to obtain a black powder sample.
[0023] According to the powder X-ray diffraction analysis results, the powder is made of La 0.9 Ce 0.1 co 0.5 Ni 0.5 o 3 Composite oxides with a perovskite structure and a small amount of CeO 2 , NiO x and CoO x composed mixture. The specific surface area is 12 m2 / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com