Precipitated aragonite and a process for producing it
a technology aragonite, which is applied in the field of precipitation, can solve the problems of high production cost of precipitated calcium carbonate of pigment grades, difficult control on an industrial scale, and slow process of aragonite production, and achieves the effects of improving the quality of aragoni
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0225] Screening Test for the Potential Active Agents:
[0226] Possible active agents were investigated by producing particulate precipitated calcium carbonate according to the following procedure:
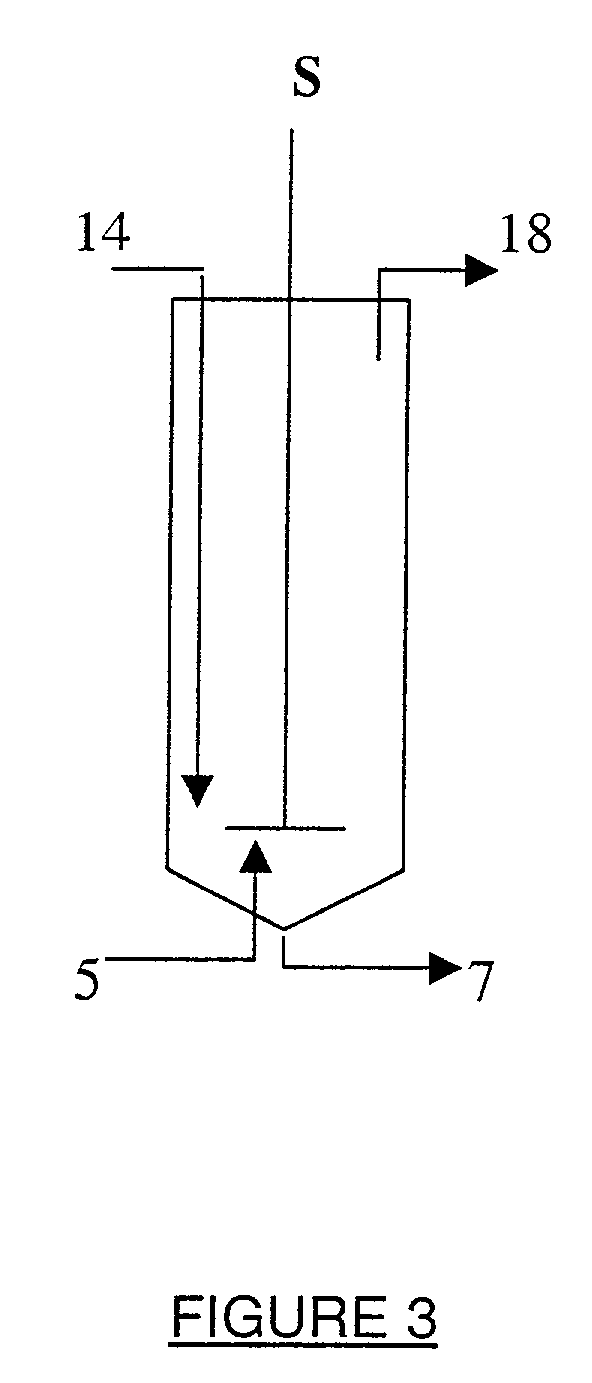
[0227] 2 kg tap water were added to a 3.2 l. stainless steel 316 reactor (of inner diameter d=15 cm and length .about.18 cm), equipped with a steam heated jacket, a pH electrode, a thermometer and the Hsiangtal Dissolver with a saw-blade rotor of d=4.8 cm (c.f. FIG. 3). The Dissolver was operated at a preset speed and carbon dioxide gas or a carbon dioxide containing gas and the aqueous calcium hydroxide slurry of PREPARATION I, containing already the active agent, were fed simultaneously into the reactor, while maintaining the pH, the temperature and the production rate at preset values. The product was collected at the top of the reactor, and the impurities were discharged from the bottom of the reactor (naturally, the product exited from the bottom of the reactor when the experimental act...
example 2
[0236] A Screening Test for Interfering Compounds:
[0237] EXAMPLE 1 was repeated, except that in all the experiments 1% (wt; based on the calcium carbonate) decanoic acid was premixed in the aqueous calcium hydroxide slurry feed and in each experiment an additional experimental active agent was added to study its effect on the activity of the decanoic acid. The results are shown in Table 2, below.
[0238] The Process Set Points--Continuous Mode of Operation:
[0239] 1. Rotor Speed=4000 rpm (Tip Speed .about.10 m / sec.)
[0240] 2. pH=9.5.
[0241] 3. Temperature=85.degree. C.
[0242] 4. Carbon dioxide flow rate=180 L.P.H. (liters / hour).
[0243] 5. Aqueous calcium hydroxide slurry (of Shfeya) -10% (wt)=.about.6 L.P.H. (to maintain the preset pH value).
[0244] 6. Active agents concentrations=1 wt. % decanoic acid+1 wt. % potential active agent based on CaCO.sub.3.
2TABLE 2 the results of EXAMPLE 2 Test Number of Product # Active Agent Carbons (Isomorph) 1 Propionic acid 3 Aragonite 2 Lactic acid 3 Arag...
example 3
[0245] A Batch Mode of Operation:
[0246] A batch mode of operation, of which parameters were as close as possible to those of EXAMPLE 1, was attempted. Only particulate precipitated calcite of rhombohedral shape was obtained. No particulate precipitated aragonite could be obtained when using decanoic acid or any other active agent that was mentioned as being effective in EXAMPLE 1. The experiment was conducted as follows:
[0247] The active agents were investigated by producing precipitated calcium carbonate particles according to the following procedure:
[0248] 2 kg aqueous calcium hydroxide slurry, containing already the respective active agent (c.f. EXAMPLE 1) were added to the 3.2 l. stainless steel 316 reactor of EXAMPLE 1. The Dissolver was operated at 4000 rpm, the temperature was maintained at 85.degree. C. and the production rate was determined by controlling the feed rate of the carbon dioxide gas. The carbonation was stopped after about 20-30 minutes, when the pH reached 7. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling temperature | aaaaa | aaaaa |
| boiling temperature | aaaaa | aaaaa |
| boiling temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com