Method of drying copper foil and copper foil drying apparatus
a drying apparatus and copper foil technology, applied in drying machines with progressive movements, lighting and heating apparatus, furnaces, etc., can solve the problems of large space required, large size of the apparatus, and large energy loss attributed to discharged hot air, and achieve high energy efficiency, easy absorbing, and improved acid resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0070] Electrodeposited copper foil having a thickness of 35 .mu.m was electrolyzed in an acidic copper sulfate solution so that the electrodposited copper foil was provided with copper plating to roughen the matte side of the electrodeposited copper foil. Thus, the copper foil having its matte side overlaid with a particulate copper layer was obtained (nodularization).
[0071] The resultant copper foil was electrolyzed in a zinc solution bath of pH 11.0 containing 10 g / L. of zinc pyrophosphate and 100 g / L. of potassium pyrophosphate at room temperature at a current density of 5 A / m.sup.2 for 6 sec so that the copper foil on its matte side was overlaid with 400 mg / m.sup.2 (in terms of zinc) of a zinc plating.
[0072] Subsequently, the zinc plated copper foil was electrolyzed in a chromating solution of pH 10 containing 2 g / L. of chromic acid at room temperature at a current density of 0.5 A / m.sup.2 for 5 sec so that the copper foil surface on its matte side was overlaid with a chromate ...
example 2
[0080] With respect to the energy required for raising the temperature of the surface of the copper foil after dewatering obtained in the same manner as in Example 1 to given level, near infrared ray, far infrared ray and hot air drying were compared to each other in the power and time spent for raising the temperature of the surface of the copper foil to given level. The results are given in Table 1 and FIG. 4.
1 TABLE 1 Index of electric energy (KWH / t) for increasing foil temp. to 130.degree. C. Near I.R. ray drying 100 Far I.R. ray drying 350 Hot air drying 250
[0081] As apparent from the results of FIG. 4, in the comparison of the time spent for raising the temperature of the surface of the copper foil to 130.degree. C., the time was only 1 sec when near infrared ray drying was used while about 15 sec was needed when far infrared ray drying was used although the far infrared heater had the same capacity as that of the near infrared heater.
[0082] Further, as apparent from the resul...
example 3
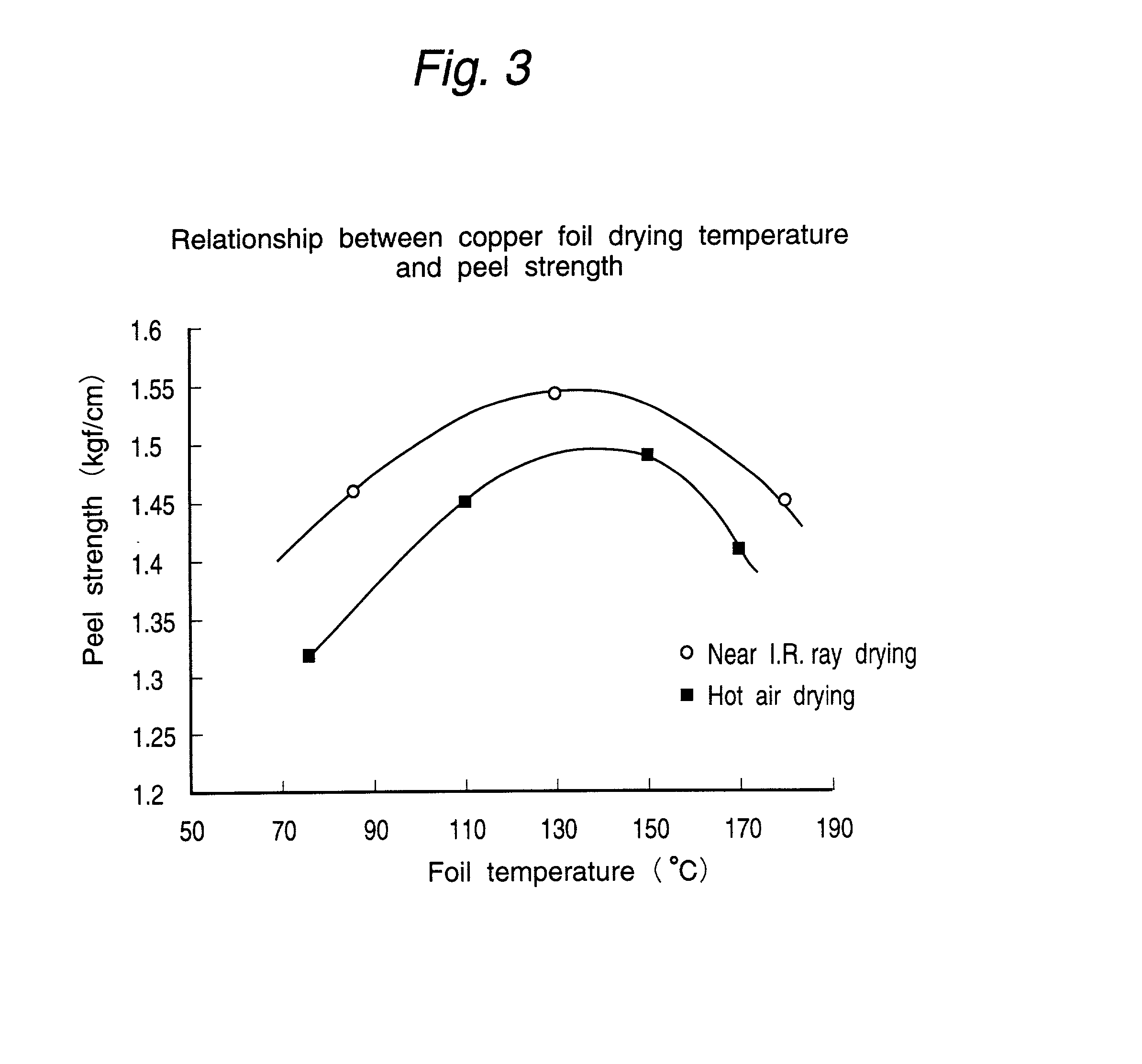
[0083] Copper foils produced by drying after dewatering in the same manner as in Example 1 with the use of near infrared rays at varied copper foil surface drying temperatures were hot-pressed to glass epoxy substrates, etched in 0.8 mm width and immersed in a 12% hydrochloric acid solution at room temperature for 30 min to thereby compare the acid resistances thereof with each other.
[0084] For comparison, the copper foils were dried by hot air at the same varied drying temperatures, and the acid resistances thereof were compared with each other.
[0085] The results are given in Table 2 below.
2 TABLE 2 Peel loss after Drying temp. (.degree. C.) HCL (%) Near I.R. ray 1 85 21 drying 2 110 6 3 150 0 4 170 12 Hot air drying 5 85 22 (Comp.) 6 110 10 7 150 6
[0086] It is apparent from the results of Table 2 that the hydrochloric acid resistance (improvement of peel loss after HCL) is enhanced by near infrared ray drying conducted with the drying temperature of the surface of the copper foil ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com