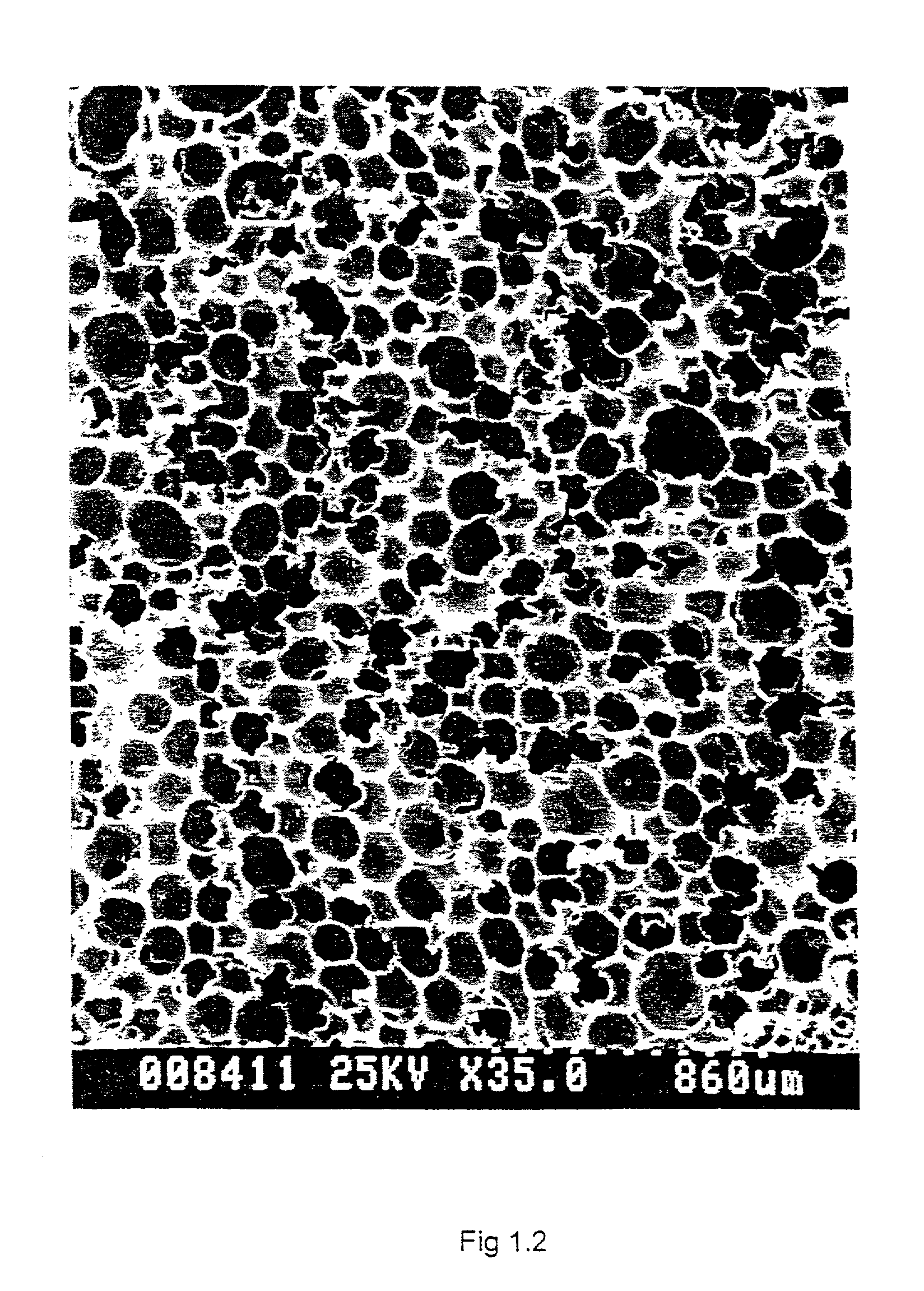
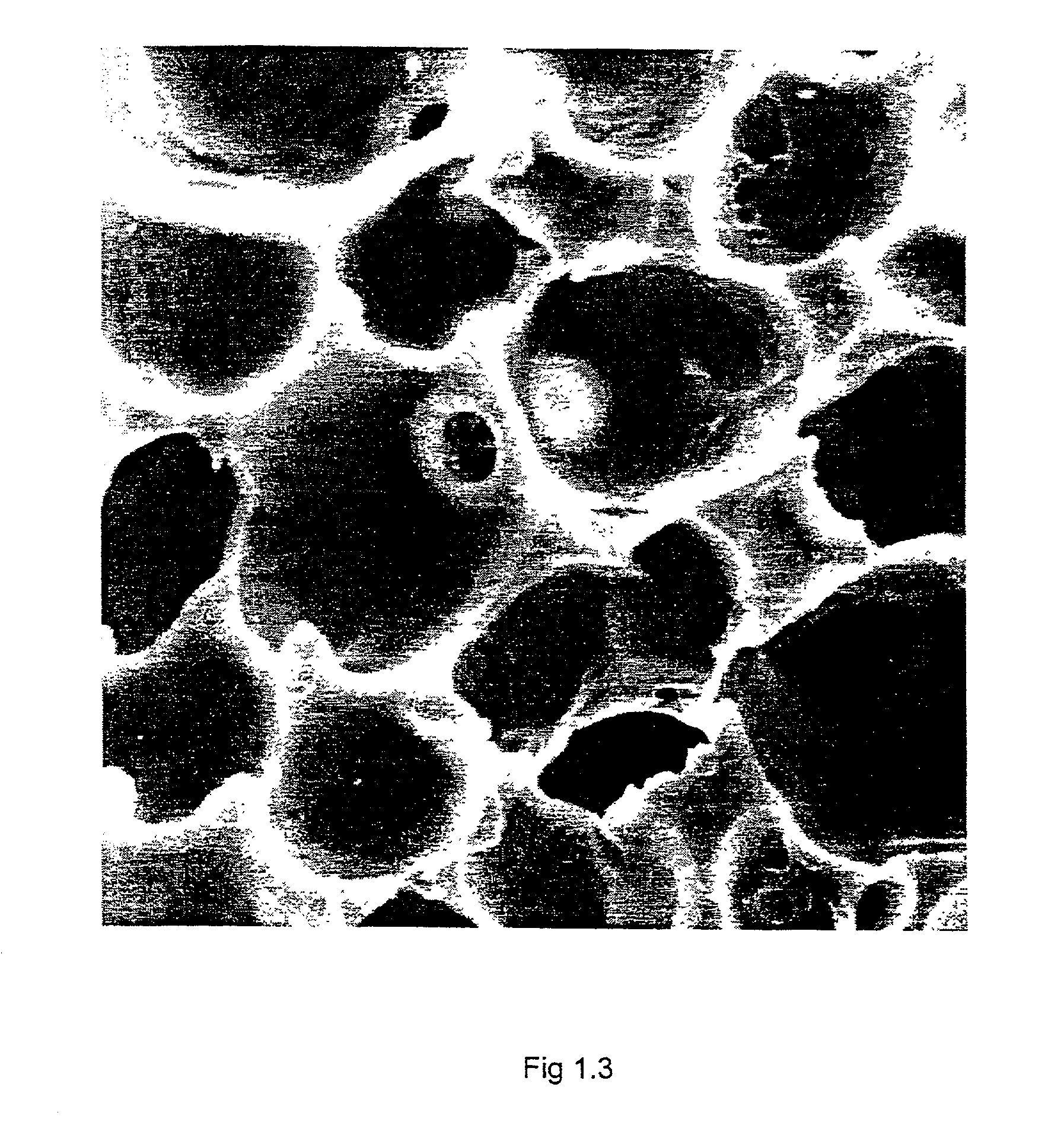
Biostable polyurethane products
a porous polyurethane and foam technology, applied in the field of biostable porous polyurethane foam implant material, can solve the problems of material ultimately affecting material and device integrity, polyether polyurethanes are not suitable candidates, and polyether polyurethanes are susceptible to phagocyte mediated environmental stress cracking, etc., and achieve the effect of strongly contributing to the hard phase stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0122] For preparation of porous biostable polytetramethylene oxide polyurethane urea isocyanurate biomaterial, a polyol resin and an isocyanate pre-polymer were prepared. In the preparation of the polyol resin the following raw materials were added to a heated round bottom flask and mixed at 50-60.degree. C. for a minimum of 25-30 minutes.
3 Raw material Quantity (php) Terathane (MW 1000).sup.1 100 Triethanolamine.sup.2 4.6 Water.sup.2 2.56 1,4 Butanediol.sup.2 8.05 BF 2270.sup.3 1.0 RC Catalyst 105.sup.4 2.96 Desmorapid PP.sup.5 0.34 Kac / Deg.sup.6 0.73 .sup.1PTMEG (Du Pont), .sup.2Sigma Aldrich, .sup.3Th GoldSchmidt, .sup.4Triethylenediamine (Rhein Chemie), .sup.5Whitchem, .sup.6Potassium acetate (Sigma Aldrich)
[0123] An isocyanate pre-polymer with an NCO content of 15.6% was prepared by charging flake MDI (Desmodur 44M flakes from Bayer, MDI-2,4' isomer content of 1.37%) into a heated round bottom flask and allowing the flakes to melt. Upon complete melting of the MDI, the PTMEG (...
example 2
[0142] For preparation of porous biostable polytetramethylene oxide polyurethane urea isocyanurate biomaterial, a polyol resin and an isocyanate pre-polymer were prepared. The polyol is prepared per example 1 using the following chemicals
4 Raw material Quantity (php) Terathane (MW 1000).sup.1 100 Triethanolamine.sup.2 4.6 Water.sup.2 3.0 1,4 Butanediol.sup.2 8.0 BF 2270.sup.3 1.2 RC Catalyst 105.sup.4 0.52 DABCO BL11.sup.5 0.13 Kac / Deg.sup.6 0.73 .sup.1PTMEG (Du Pont), .sup.2Sigma Aldrich, .sup.3Th GoldSchmidt, .sup.4Triethylenediamine solution (Rhein Chemie), .sup.5Air Products, .sup.6Potassium acetate solution (Sigma Aldrich)
[0143] An isocyanate pre-polymer with a NCO content of 25.0% was prepared as per example 1 using PTMEG polyol (Terathane MW 650).
[0144] The polytetramethylene oxide polyurethane urea isocyanurate biomaterial was prepared per the method of example 1 at an isocyanate index of 1.13 with the exception that the combined shot size of the components was 1.3 g
[0145] R...
example 3
[0147] For preparation of a porous biostable polycarbonate polyurethane urea isocyanurate biomaterial, a polyol resin and an isocyanate pre-polymer were prepared.
[0148] The polyol is prepared per example 1 using the following chemicals.
5 Raw material Quantity (php) Polycarbonate CX 5510 (MW 1000).sup.1 100 Triethanolamine.sup.2 3.6 Water.sup.2 3.0 BF 2270.sup.3 1.2 RC Catalyst 105.sup.4 1.66 DABCO BL 11.sup.5 0.8 Kac / Deg.sup.6 0.73 .sup.1Nissei Chemical Company, Japan. .sup.2Sigma Aldrich .sup.3Th GoldSchmidt .sup.4Triethylene diamine solution (Rhein Chemie) .sup.5Air Products .sup.6Potassium acetate solution (Sigma Aldrich)
[0149] The isocyanate pre-polymer was prepared as per example 1 using polycarbonate CX 5510 (MW 1000) to produce a prepolymer with an isocyanate content of 15.6%
[0150] Polycarbonate CX5510 is a random co-polymer comprising hexamethylene carbonate and pentamethylene carbonate sequences of 1000MW.
[0151] The materials were mixed at an isocyanate index of 1.13 in an ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com