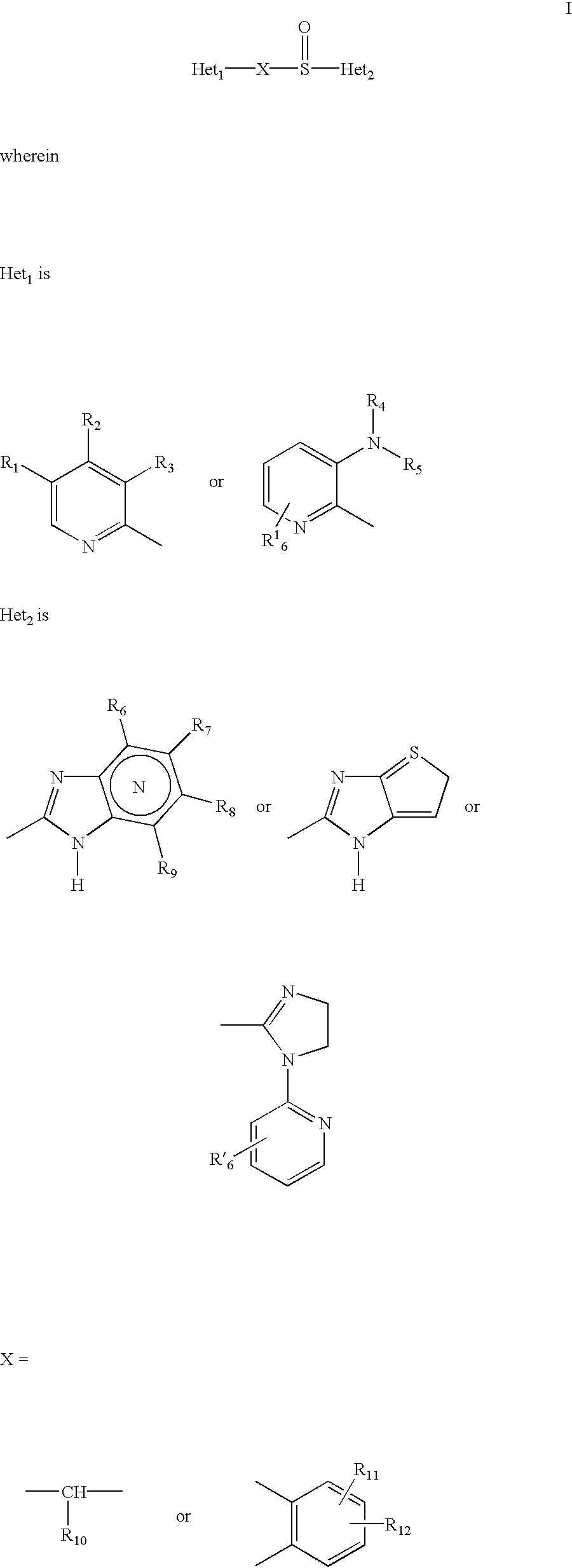
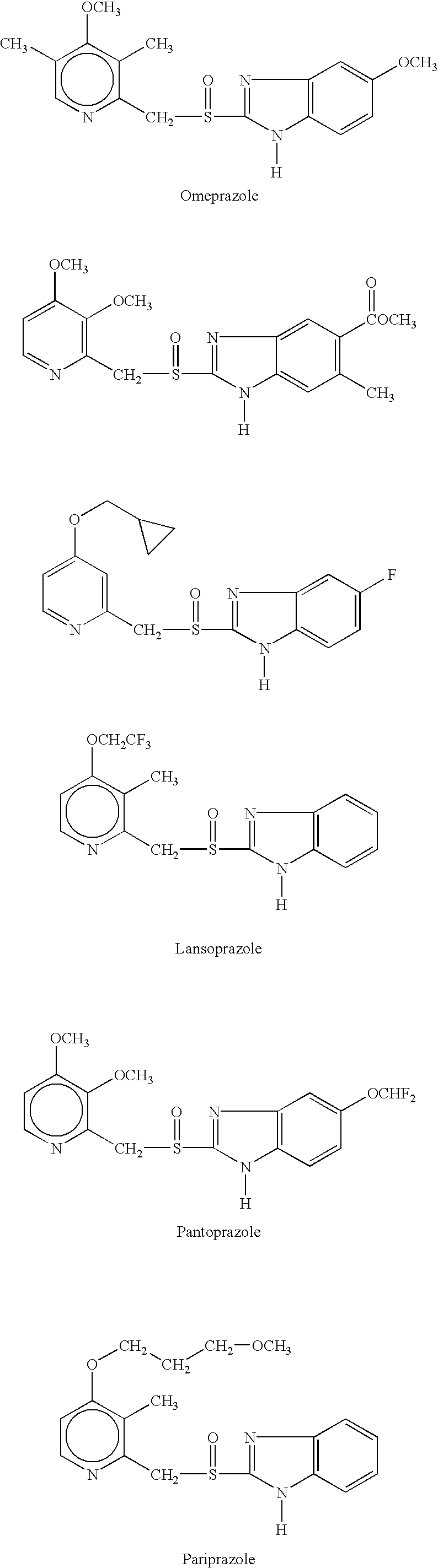
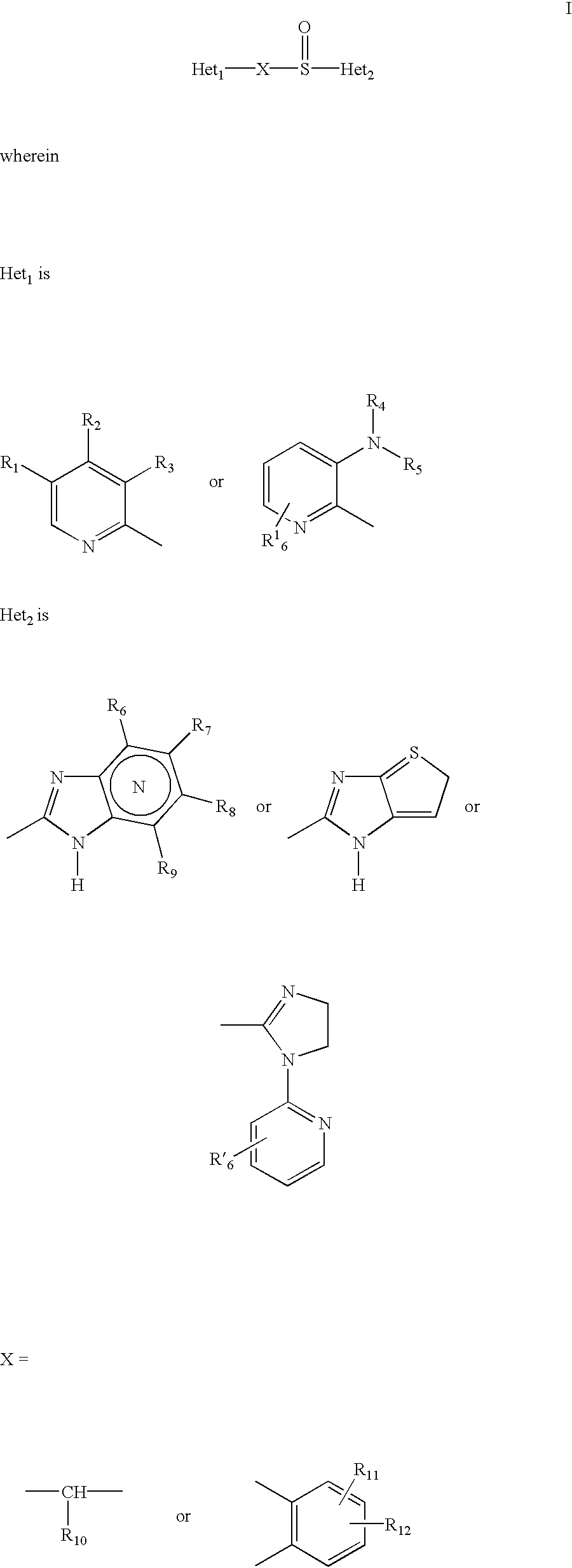
Pharmaceutical formulation and process
a technology of pharmaceutical formulation and process, applied in the field of new pharmaceutical formulation, can solve the problems of discoloration of active compound content and loss of content with time, and the inability to apply at least two different layers, and achieve the effect of simplifying the preparation of enteric coated articles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0046] Tablets containing lansoprazole and arginine are produced according to the following procedure. Firstly, dry ingredients are thoroughly mixed and then granulated with a solution in a laboratory mixer. The dried granules are mixed with lubricants etc. in a final mixing step.
1 Concentration Dry ingredients for granulation (mmol / g dry ingredients in (for approx. 4000 tablets) the alkaline tablet core) Lansoprazole 40.4 g L-arginine (passing 120 mesh) 365.4 g 4.2 Microcrystalline cellulose 38.5 g Granulating solution Distilled water 173 g Corn starch 7.7 g
[0047] The solution is poured over the premixed powder mass during mixing. The wet granules are dried on a tray in a drying cabinet. The dried granules are milled to pass a 1.0 mm sieve. The granules are mixed with
2 Talc 3.1 g Sodium dodecyl sulphate 20.8 g Microcrystalline cellulose 19.2 g Magnesium stearate 5.0 g
[0048] in a laboratory mixer, and then compressed into tablets having a size of 7 mm .O slashed. and a weight of app...
example 2
[0052] Core material containing the magnesium salt of (-)-omeprazole and the alkaline reacting compound trometamine (=tris-buffer) is prepared by extrusion and spheronization.
[0053] The powder mass is mixed in a laboratory mixer and then water is added.
4 Concentration (mmol / g dry ingredients in the Powder mixture alkaline core material) Magnesium salt of (-)-omeprazole 400 g Microcrystalline cellulose 300 g Trometamine 1000 g 4.1 PVP-XL 100 g Mannitol pwd 195 g Hydroxypropyl methylcellulose 6 cps 5 g Water q.s.
[0054] The powder mixture is mixed with the water and the wet mass is mixed to obtain a suitable consistency of the mass.
[0055] Extrusion is performed with an extruder fitted with 1.0 mm screen. The extrudate is formed into pellets on a spheronizer and dried in a fluidized bed drier.
[0056] 200 g of the obtained pellets are spray coated with the enteric coating dispersion described below, in a Wurster equipped fluidized bed.
5 Enteric coating dispersion Water 93.9 g Polyethylene...
example 3
[0059] Core material containing omeprazole and N-methyl-D-glucamine (=meglumine) is prepared by extrusion and spheronization of the below described composition using the same procedure as in Example 2;
6 Concentration (mmol / g dry ingredients in the Powder mixture alkaline core material) Omeprazole 100.0 g Microcrystalline cellulose 50.0 g Meglumine 500.0 g 2.6 Mannitol pwd 297.0 g Sodium starch glycolate 48.0 g Sodium laurylsulphate 5.0 g Water q.s.
[0060] Obtained dried pellets / cores are spray coated with the enteric coating dispersion described below, in a Wurster equipped fluidized bed.
7 Enteric coating dispersion Water 93.9 g Polyethylene glycol 400 4.6 g Eudragit .TM. L30D-55 151.5 g
[0061] This single coating step resulted in tablets having two polymeric layers with different characteristics. The inner layer is not soluble in acetone, as the outer one, but soluble in water. The separating layer is spontaneously formed in situ during the process, as a salt between the alkaline rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com