Insoluble compositions for controlling blood glucose
a composition and insoluble technology, applied in the human field, can solve the problems of life-threatening hypoglycemia, inability to provide ideal "flat" pharmacokinetics for maintaining optimal fasting blood glucose, and inability to achieve the ideal "flat" pharmacokinetics of nph insulin preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
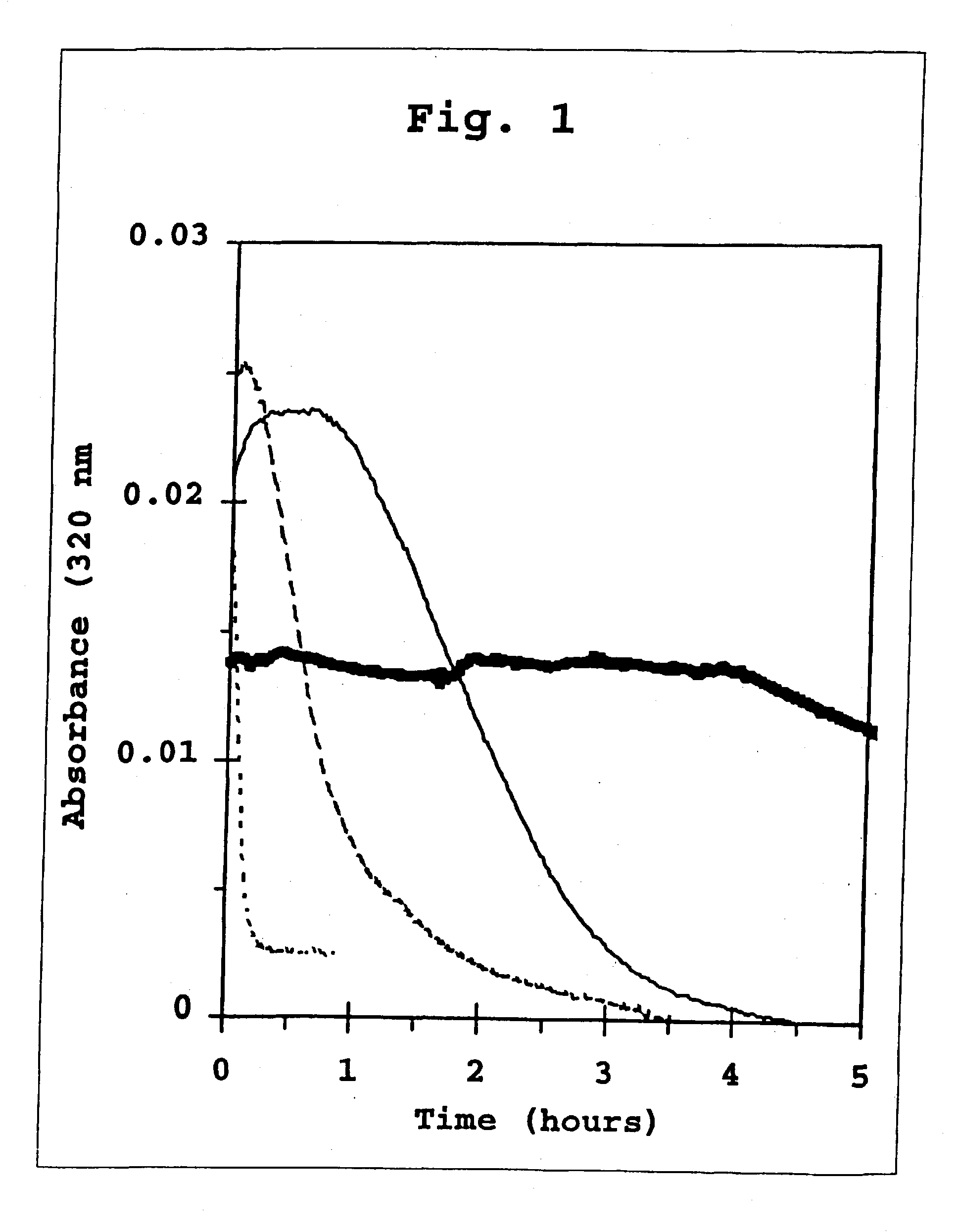
preparation 1
9:1 Co-Crystals of Human Insulin and B29-N.epsilon.-octanoyl-Human Insulin
[0414] A dry powder of B29-N.epsilon.-octanoyl-LysB29 human insulin (0.7 parts by mass) and a dry powder of humen insulin (6.3 parts by mass) are dissolved in 1000 parts by volume of an aqueous solvent composed of 50 mM TRIS, 0.1 M trisodium citrate, and 10 mg / ml phenol at pH 7.6. To this solution is added 75 parts of a 15.3 mM solution of zinc chloride. The pH is adjusted to 7.6 with 1 N HCl and / or 1 N NaOH. This solution is filtered through a 0.22 micron, low-protein binding filter. A second solution is prepared by dissolving 7 parts by mass of protamine sulfate in 10,000 parts by volume of water then filtering through-a 0.22 micron, low-protein binding filter. Equal volumes of the solution containing insulin and acylated insulin and of the protamine sulfate solution are combined. Initially, an amorphous precipitate forms. This suspension is allowed to stand for about 24 hours at room temperature (typically ...
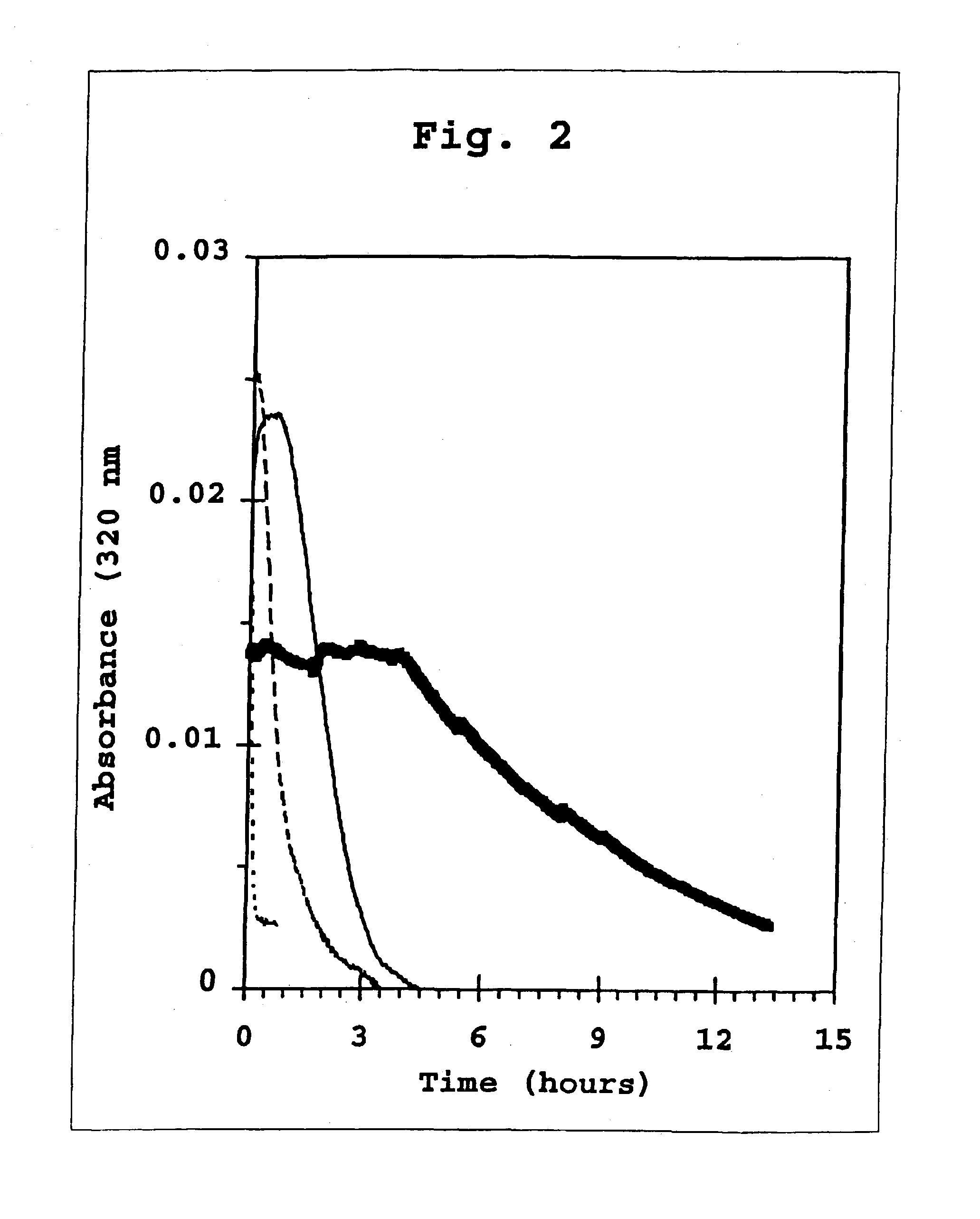
preparation 2
3:1 Co-Crystals of Human Insulin and B29-N.epsilon.-octanoyl-Human Insulin
[0415] The procedure of Preparation 1 is followed, except that 1.75 parts by mass of a dry powder of B29-N.epsilon.-octanoyl-LysB29 human insulin and 5.25 parts by mass of a dry powder of human insulin are used. After equal volumes of the solution containing insulin and acylated insulin and of the protamine sulfate solution are combined, an amorphous precipitate forms. This suspension is allowed to stand for about 24 hours at room temperature (typically about 22.degree. C.). The amorphous precipitate converts to a co-crystalline microcrystalline solid.
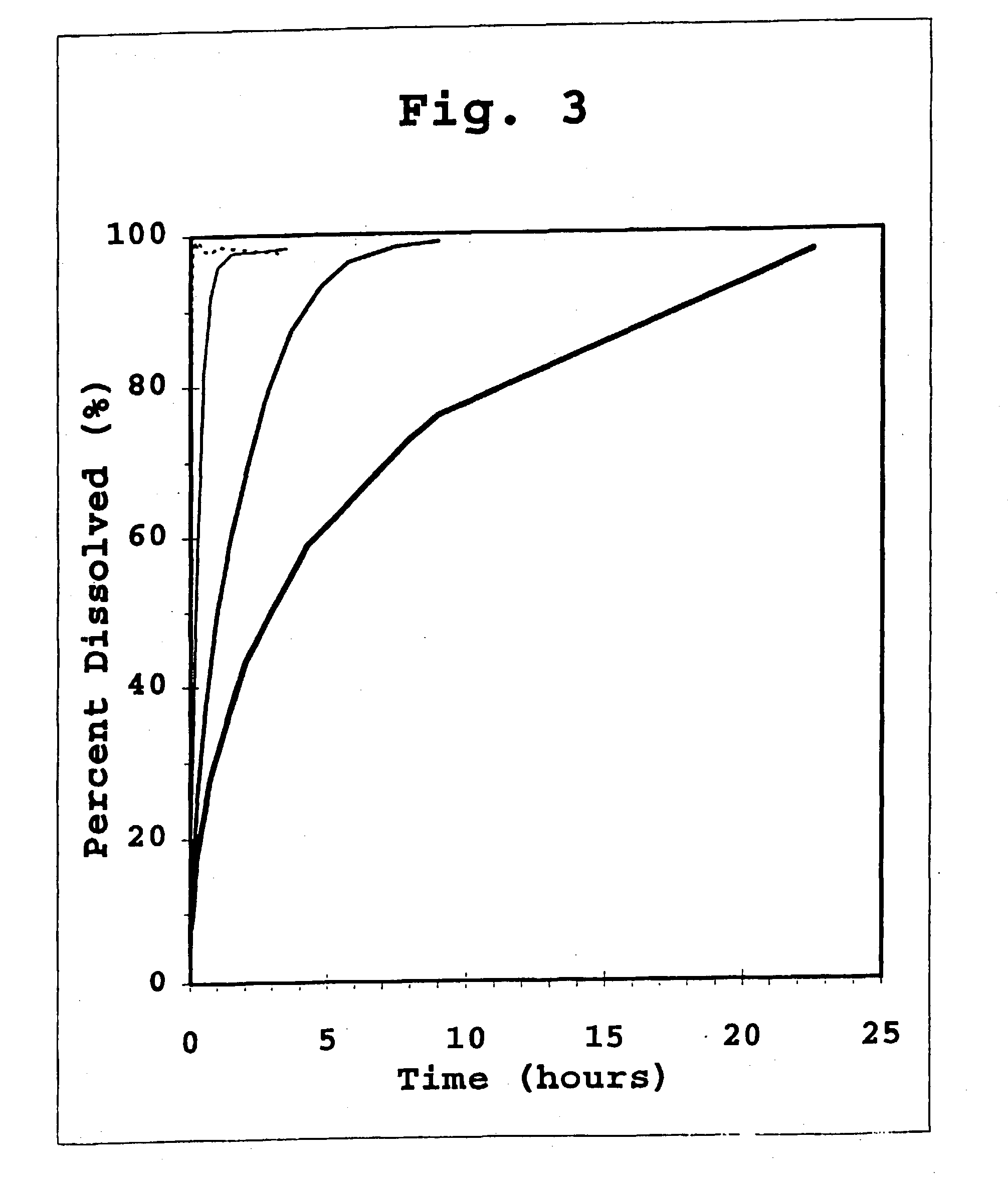
preparation 3
Formulation of 3:1 Co-Crystals of Human Insulin and B29-N.epsilon.-octanoyl-Human Insulin
[0416] The co-crystalline microcrystals prepared by the method of Preparation 1 are separated from the supernatant and are recovered by conventional solid / liquid separation methods, such as, filtration, centrifugation, or decantation. The recovered co-crystalline microcrystals are then suspended in a solution consisting of 25 mM TRIS, 5 mg / ml phenol, and 16 mg / ml glycerol, pH 7.8, so that the final concentration of insulin activity is about.100 U / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mole ratio | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com