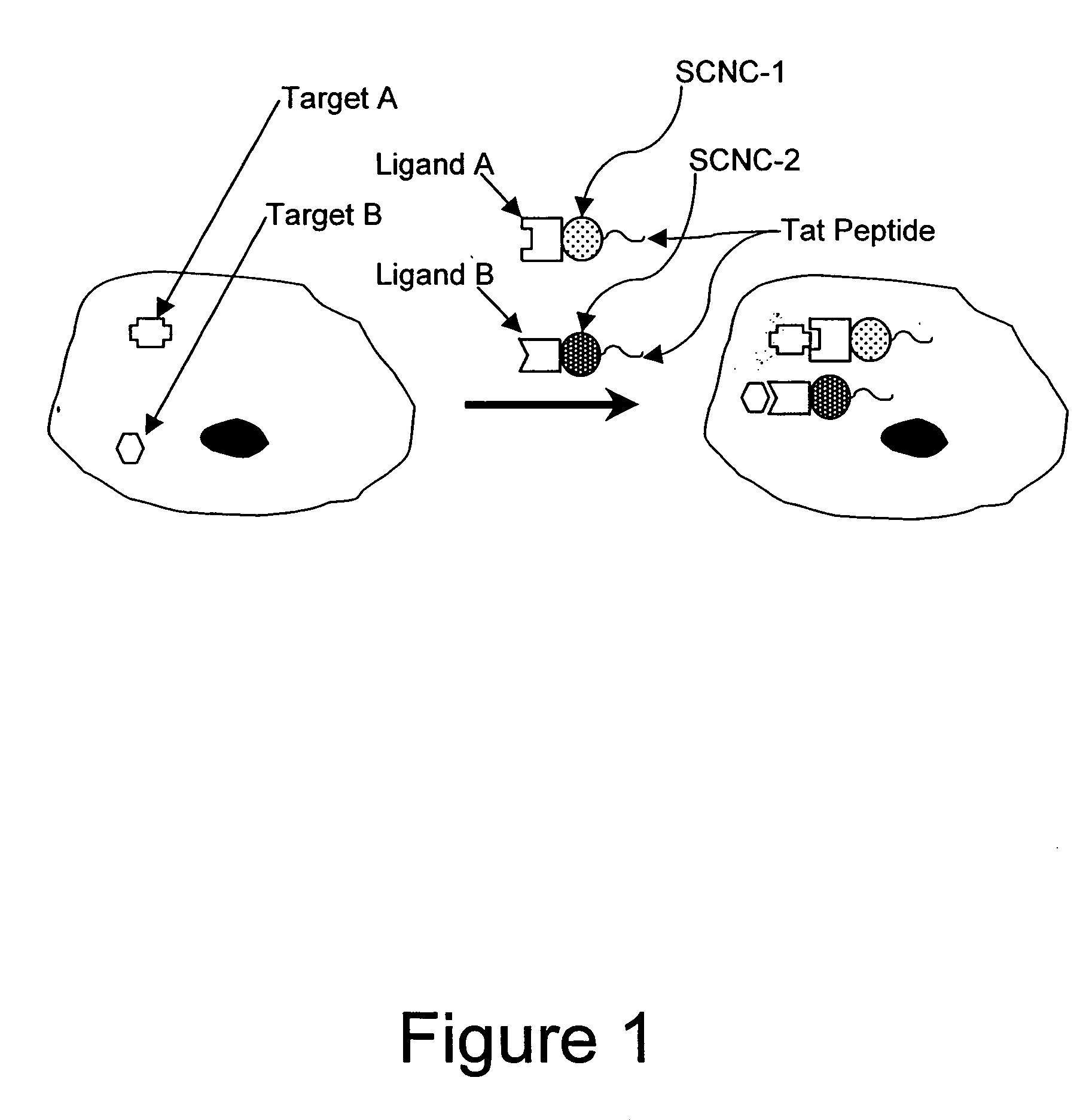
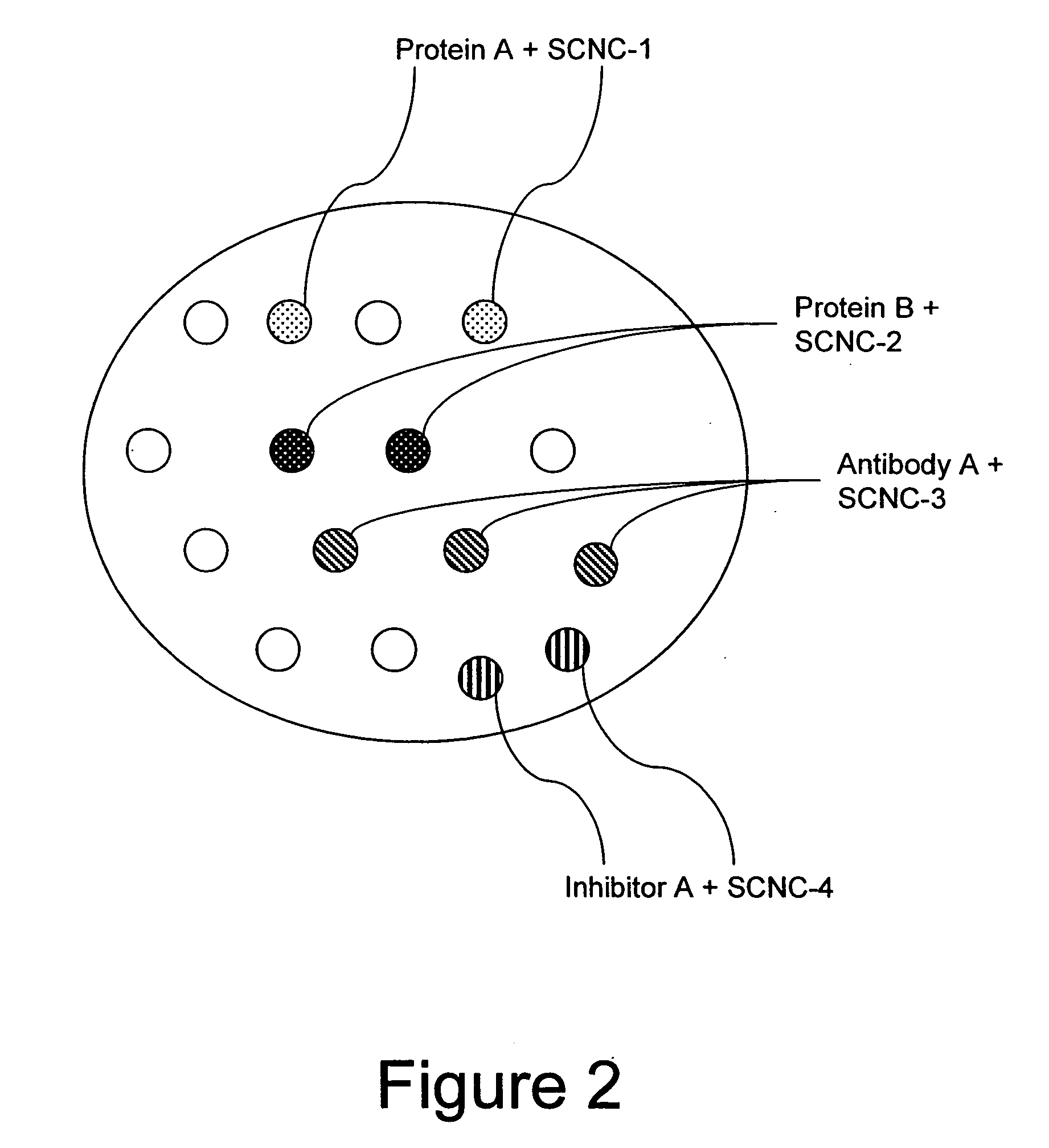
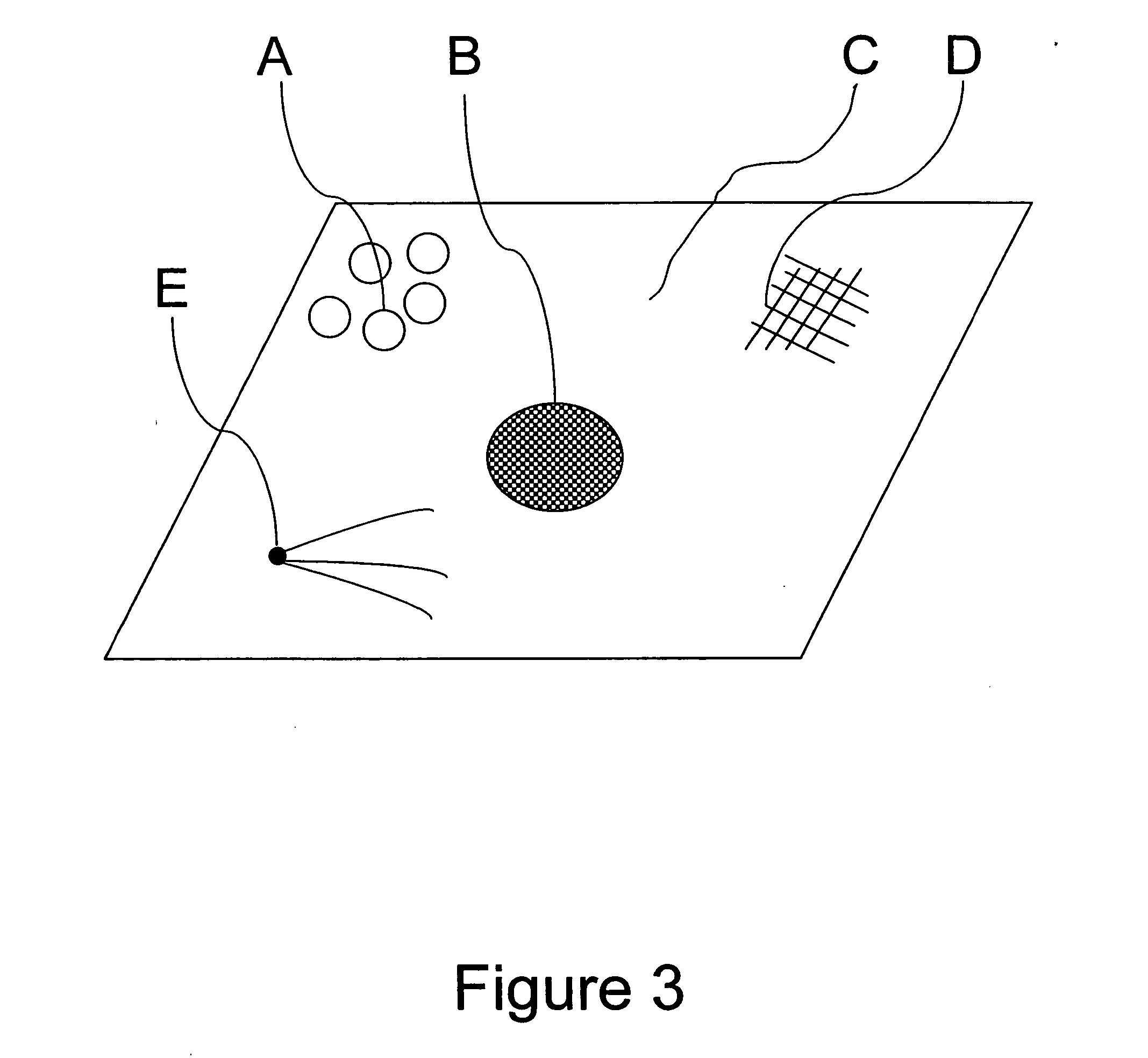
Method for enhancing transport of semiconductor nanocrystals across biological membranes
a technology of semiconductor nanocrystals and biological membranes, applied in the field of semiconductor nanocrystal probes, can solve the problems of complex robotics and fluid dispensing systems, affecting the optimal functioning of fluid delivery and evaporation of assay solution at this scale, and limiting the flexibility of each format, so as to facilitate analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Peptide-mediated Uptake of SCNCs
[0344] Chariot (Active Motif, Carlsbad, Calif.) is a peptide reagent based on the HIV-tat sequence (Schwarze et al. (1999) Science 285:1569-1572), and has been used to deliver a variety of macromolecules into cells. Chariot forms a non-covalent complex with a molecule of interest (protein, peptide, antibody, or SCNC), and acts as a carrier to deliver molecules into cells.
[0345] To deliver SCNCs into cells using Chariot, tissue culture cells were seeded into six-well tissue culture plates (surface area of 962 mm2 per well) at a cell density of 3×105 cells per well and incubated overnight at 37° C. in a 5% CO2 atmosphere. The transfection efficiency was dependent on the percent confluency of cells; the optimal percent confluency for Chinese Hamster Ovary (CHO) cells was about 50-70%.
[0346] The transfection mixture was prepared by first diluting 616 nm emitting SCNCs into PBS in a final volume of 100 μl. The diluted SCNCs were combined with a mixture ...
example 2
Nonspecific Uptake of SCNCs
[0348] SCNCs can be internalized by cells in the absence of a specific carrier molecule. Non-crosslinked polymer-coated SCNCs prepared as described above are sufficiently hydrophobic that they bind to cells and are taken up by nonspecific endocytotic pathways. Cells encoded with SCNCs were prepared as described in Example 1, except the Chariot reagent was omitted from the transfection mix. An example of nonspecific uptake of SCNCs is shown in FIG. 11.
example 3
Cationic Lipid-mediated and Micelle-mediated Uptake of SCNCs
[0349] BioPORTER (BioPORTER, Gene Therapy Systems, San Diego, Calif.) is a cationic lipid that is similar to other lipid-based reagents for DNA transfections. It forms ionic interactions with negatively charged groups of a molecule (protein, peptide, antibody, or SCNC), and delivers the molecule into cells via fusion with the cell membrane.
[0350] Cells were seeded at the same density as described in Example 1. A transfection mix, comprised of carboxylated SCNCs and PBS in a final volume of 100 μl, was added to a tube containing 10 μl of dried BioPORTER reagent. The solution was mixed gently by pipetting, incubated at room temperature for 5 minutes, and diluted by adding 900 μl of serum free medium. Cells were washed with PBS, and the diluted SCNC solution (1 ml) was added to the cell monolayer. The final SCNC concentration was 2-60 nM, depending on the cell line and SCNC material being tested. The cells were incubated at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Digital information | aaaaa | aaaaa |
| Digital information | aaaaa | aaaaa |
| Digital information | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com