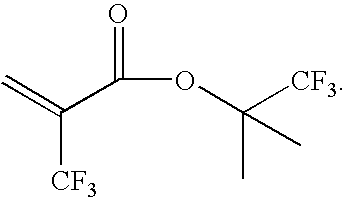
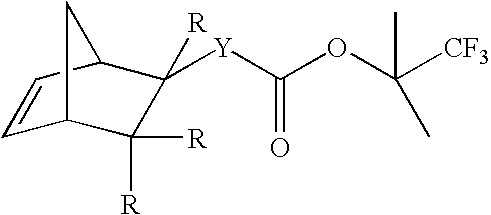
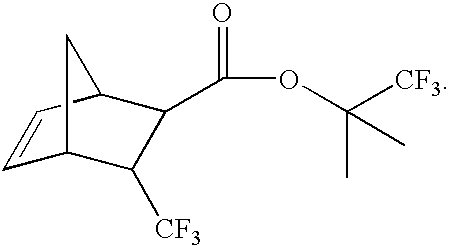
Monomers for photoresists bearing acid-labile groups of reduced optical density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of lithium tert-trifluoromethyl butoxide
[0054] Trifluoromethyl-t-butanol (40.96 g, 0.32 mole) was dissolved in 100 mL of anhydrous THF and cooled to below 5° C.; n-Butyl lithium (2.5 M in hexane, 128 mL, 0.32 mol) was added at a rate to keep the internal reaction temperature below 5° C. After the addition was complete, the reaction was stirred at room temperature for 1 h to complete the formation of the lithium salt. The conversion was quantitative. The reagent was used directly without further characterization.
example 2
Preparation of 3-trifluoromethyl-bicyclo[2.2.1]hept-5-ene-2-carboxylic acid
[0055] 3-trifluoromethyl-bicyclo[2.2.1]hept-5-ene-2-carboxylic acid ethyl ester (100 g, 0.427 mol) was added to a flask containing NaOH (85 g, 2.12 mol) in 1.5 L of H2O. The two-phase system was heated at 95° C. for 4 h, after which time, a yellow homogeneous solution resulted. The reaction was cooled to ambient temperature and extracted with hexane (2×100 mL) to remove any remaining organic species. The aqueous phase that resulted from this process was cooled to 5° C., then acidified with 12 N HCl to a pH of 1-2. The white precipitate that formed was filtered, washed with H2O (100 mL) and dried. The yield of acid was 86.83 g (98%). Melting point=85-90° C. 19F NMR: −68 ppm (d, J=9.7 Hz,exo) and −66.7 ppm (d, J=9.7 Hz,endo).
example 3
Preparation of 3-trifluoromethyl-bicyclo[2.2.1]hept-5-ene-carbonyl chloride
[0056] The norbornene acid prepared in Example 2 (75 g, 0.364 mol) was refluxed with thionyl chloride (150 mL, 2.0 mol) for 2.5 h. Excess SOCl2 was removed by distillation at atmospheric pressure. The product was isolated by vacuum distillation. Yield of colorless liquid was 73.47 g (89.9%), bp 40-43° C. / 1.0 mm. 19F NMR: −68.3 ppm (d, J=9.7 Hz,exo); −66.9 ppm (d, J=8.6 Hz,endo).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com