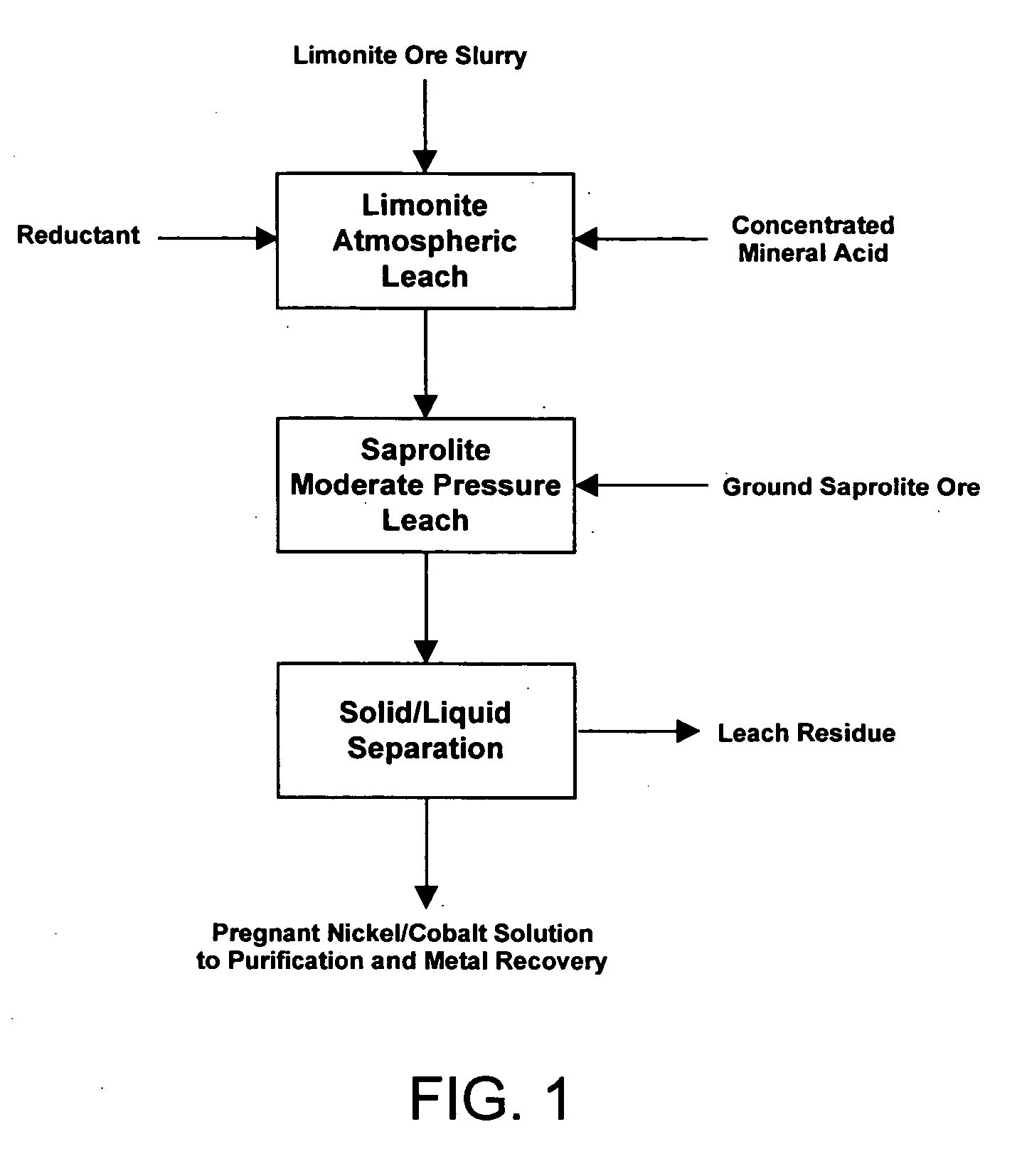
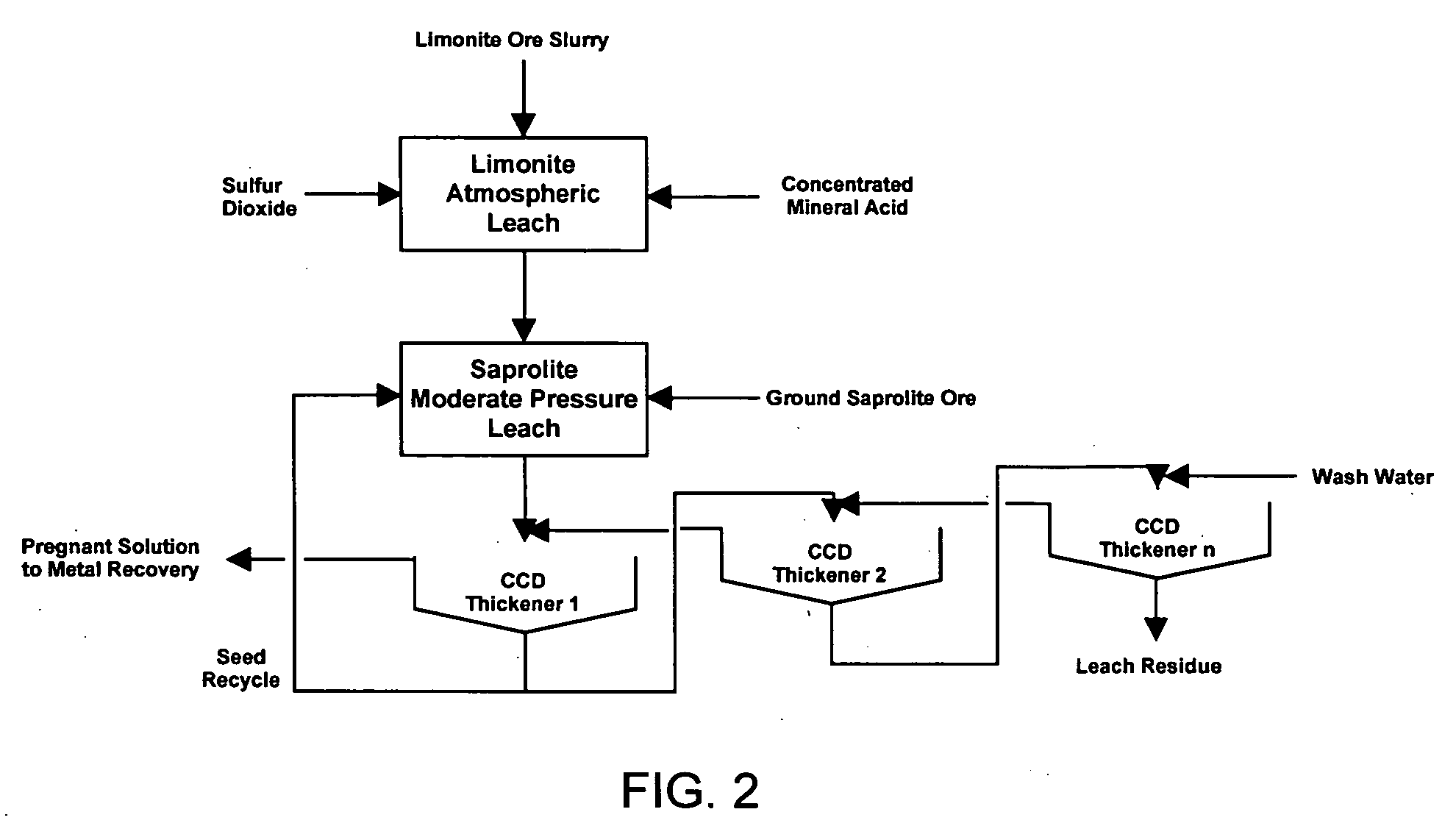
Method for nickel and cobalt recovery from laterite ores by combination of atmospheric and moderate pressure leaching
a technology of laterite and cobalt, which is applied in the direction of nickel compounds, cobalt compounds, iron compounds, etc., can solve the problems of high processing cost, inability to concentrate nickel values substantially through physical means, and high cost of processing of laterites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0055] An acid solution was prepared by adding 287.6 g of 96% sulfuric acid and 48.0 g of 37% HCl (both mineral acids being reagent grade) to 702 mL of water. The acid solution was transferred to a 2-liter cylindrical reaction kettle equipped with a mechanical stirrer, 4 plastic baffles, and a tight-fitting lid equipped with a water condenser open to the atmosphere. The reaction kettle was heated by an external, electrical heating mantle. 381.7 g (wet) of the limonite ore described in Table 1 were added to the acid solution while heating and stirring the mixture. The temperature was controlled at 94 to 105° C. and the limonite ore was leached for 5 hours. Liquid samples were taken from the leach slurry at 1, 2 and 5 hours.
[0056] After 5 hours of leaching, the leach slurry was transferred to a 2-liter titanium autoclave, along with 344.8 g (wet) of the saprolite ore described in Table 1. The saprolite ore had first been wet ground to approximately −100 mesh and then filtered to form...
example 2
[0062] Another test was carried out similarly to that described in Example 1 with the following exceptions. The limonite ore used had the following composition: 1.55% Ni, 48.4% Fe, 0.47% Mg, and 37.2% moisture. 398.1 g (wet) of this ore was used in the test, along with 573.7 g water, 288.2 g of 96% H2SO4, 46.9 g of 37% HCl, and 285.7 g of MgSO4*7H2O. No hematite seed material was used in the test. The MgSO4*7H2O was dissolved in the water prior to adding the acid to the solution. The purpose of adding soluble magnesium to the solution was to simulate the recycle of a magnesium-rich solution which would remain after nickel and cobalt recovery from the pregnant leach solution. The atmospheric leach was carried out with a temperature of 96 to 101° C. No sample was taken from the autoclave during the pressure leach stage.
[0063] The assays of the liquid samples taken during the atmospheric limonite leach are shown in Table 4 and the final solution and residue assays, as well as the calc...
example 3
[0067] For comparison, another test was done to simulate the conditions of the process described in WO 03 / 093517 A1. The atmospheric limonite leaching stage was carried out similarly to the limonite leach stage described in Example 1. 398.1 g (wet) of the same limonite ore used in Example 2 was added to a solution comprised of 719.9 g water, 288.2 g 96% H2SO4 and 46.9 g of 37% HCl. Leaching was carried out for 4 hours at 101-104° C. After 4 hours, 310.6 g of ground saprolite containing 20.0% H2O and 128.6 g hematite seed were added to the leach slurry and leaching was continued at 98-102° C. for 10 additional hours. Liquid samples were taken periodically to follow the course of leaching. The final leach slurry was filtered, the filtercake repulped twice with fresh water and the filtrate, wash solution and final washed residue were assayed as in the previous examples.
[0068] The conditions of this test were thus essentially identical to those of Example 1, except that instead of pres...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com