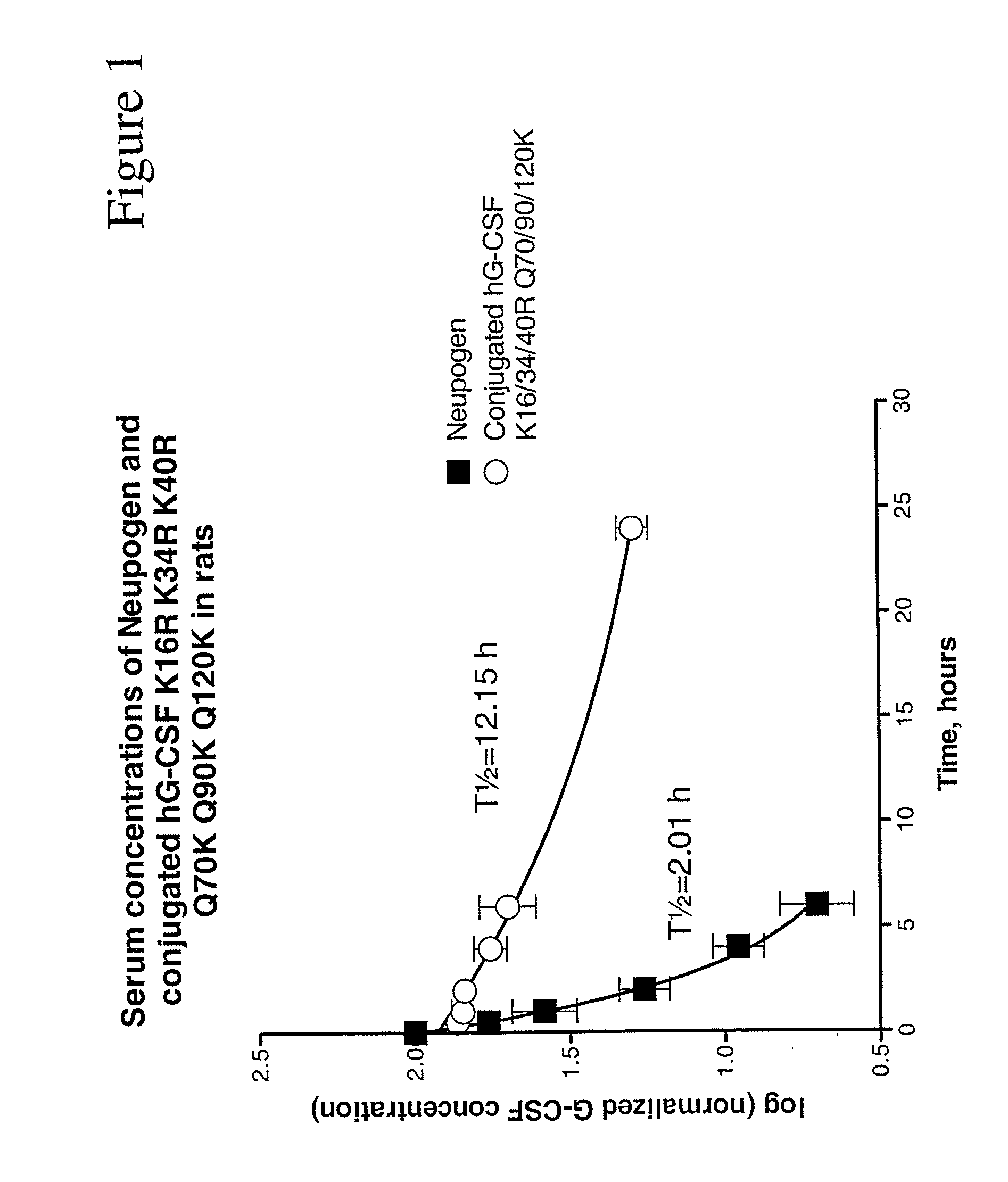
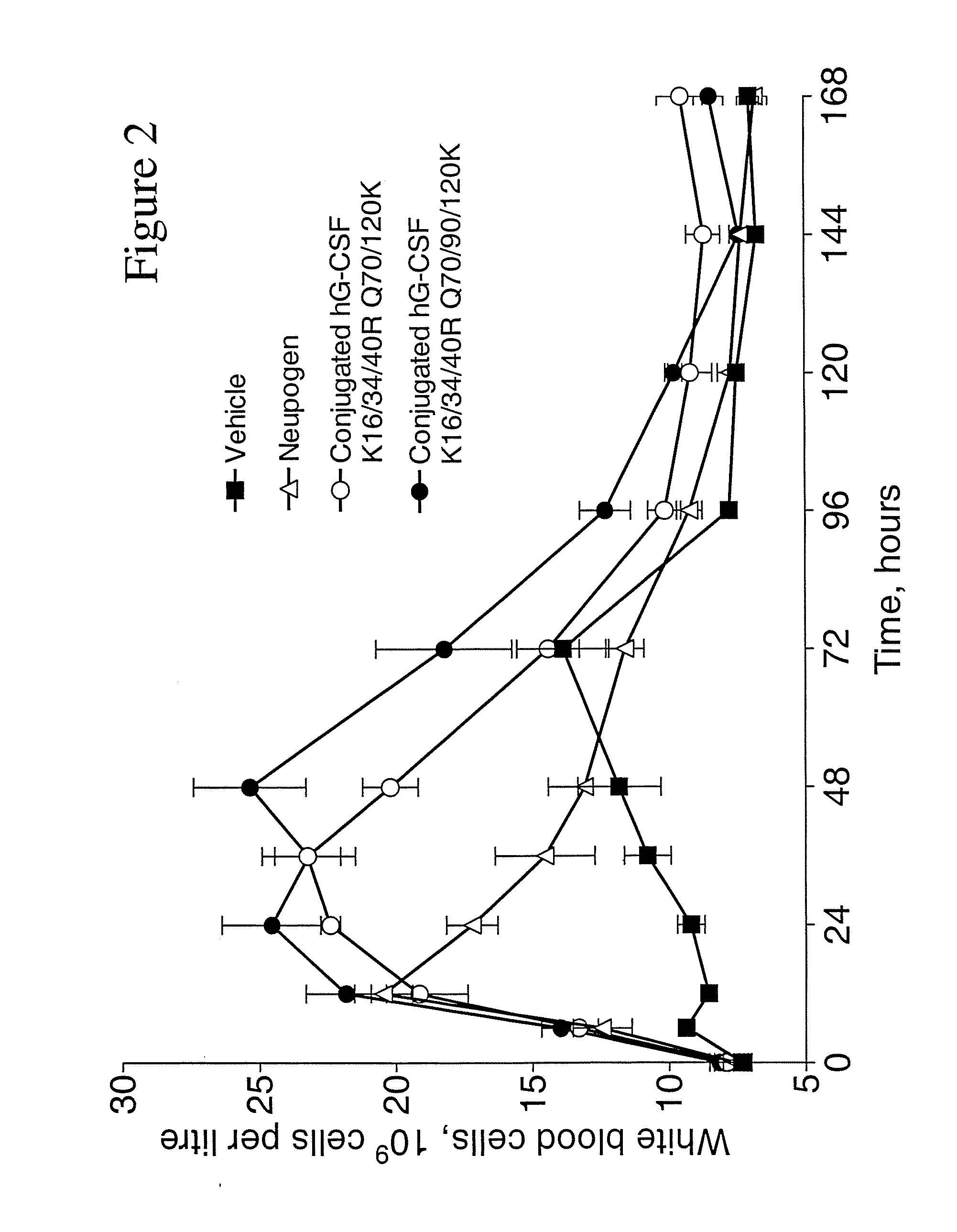
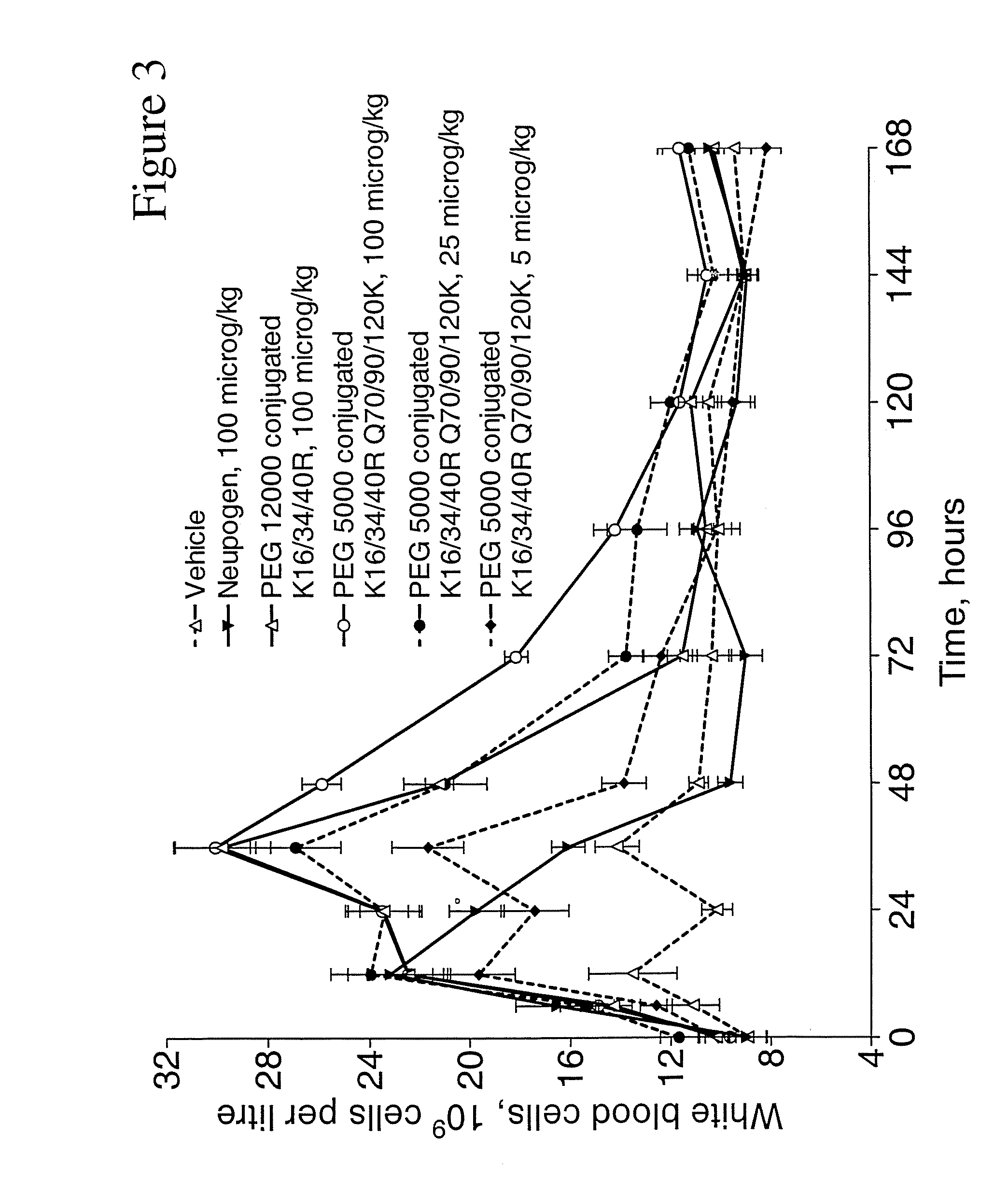
G-CSF Conjugates
a granulocyte colony-stimulating factor and conjugate technology, applied in the field of new polypeptides exhibiting granulocyte colony-stimulating factor (gcsf), can solve the problems of dose-dependent bone pain and the above-mentioned problem, and achieve the effects of improving serum half-life, improving properties, and improving serum half-li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction and Cloning of Synthetic Genes Encoding HG-CSF
[0271] The following DNA fragments were synthesised following the general procedure described by Stemmer et al. (1995), Gene 164, pp. 49-53:
[0272] Fragment 1, consisting of a Bam HI digestion site, a sequence encoding the YAP3 signal peptide (WO 98 / 32867), a sequence encoding the TA57 leader sequence (WO 98 / 32867), a sequence encoding a KEX2 protease recognition site (AAAAGA), a sequence encoding hG-CSF with its codon usage optimised for expression in E. coli, (SEQ ID NO:2) and a Xba I digestion site.
[0273] Fragment 2, consisting of a Bam HI digestion site, a sequence encoding the YAP3 signal peptide (WO 98 / 32867), a sequence encoding the TA57 leader sequence (WO 98 / 32867), a sequence encoding a histidine tag (SEQ ID NO:5), a sequence encoding a KEX2 protease recognition site (AAAAGA), a sequence encoding hG-CSF with its codon usage optimised for expression in E. coli, (SEQ ID NO:2) and a Xba I digestion site.
[0274] Frag...
example 2
Expression of hG-CSF in S. cerevisiae and E. coli
[0279] Transformation of Saccharomyces cerevisiae YNG318 (available from the American Type Culture Collection, VA, USA as ATCC 208973) with either plasmid pG-CSFcerevisiae or pHISG-CSFcerevisiae, isolation of transformants containing either of the two plasmids, and subsequent extracellular expression of hG-CSF without and with the EHS tag, respectively, was performed using standard techniques described in the literature. Transformation of E. coli BL21 (DE3) (Novagen, Cat. No. 69387-3) with pG-CSFcoli, isolation of transformants containing the plasmid and subsequent expression of hG-CSF in the supernatant and in the periplasm of the cell was performed as described in the pET System Manual (8′ edition) from Novagen.
[0280] Expression of hG-CSF by S. cerevisiae and E. coli was verified by Western Blot analysis using the ImmunoPure Ultra-Sensitive ABC Rabbit IgG Staining kit (Pierce) and a polyclonal antibody against hG-CSF (Pepro Tech E...
example 3
Purification of HG-CSF and Variants Thereof from S. cerevisiae Culture Supernatants
[0282] Purification of hG-CSF was performed as follows:
[0283] Cells are removed by centrifugation. Cell depleted supernatant is then filter sterilised through a 0.22 μm filter. Filter sterilised supernatant is diluted 5 fold in 10 mM sodium acetate pH 4.5. pH is adjusted by addition of 10 ml concentrated acetic acid per 5 liters of diluted supernatant. The ionic strength should be below 8 mS / cm before application to the cation exchange column.
[0284] Diluted supernatant is loaded at a linear flow rate of 90 cm / h onto a SP-sepharose FF (Pharmacia) column equilibrated with 50 mM sodium acetate, pH 4.5 until the effluent from the column reaches a stable UV and conductivity baseline. To remove any unbound material, the column is washed using the equilibration buffer until the effluent from the column reaches a stable level with respect to UV absorbance and conductivity. The bound G-CSF protein is eluted...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com