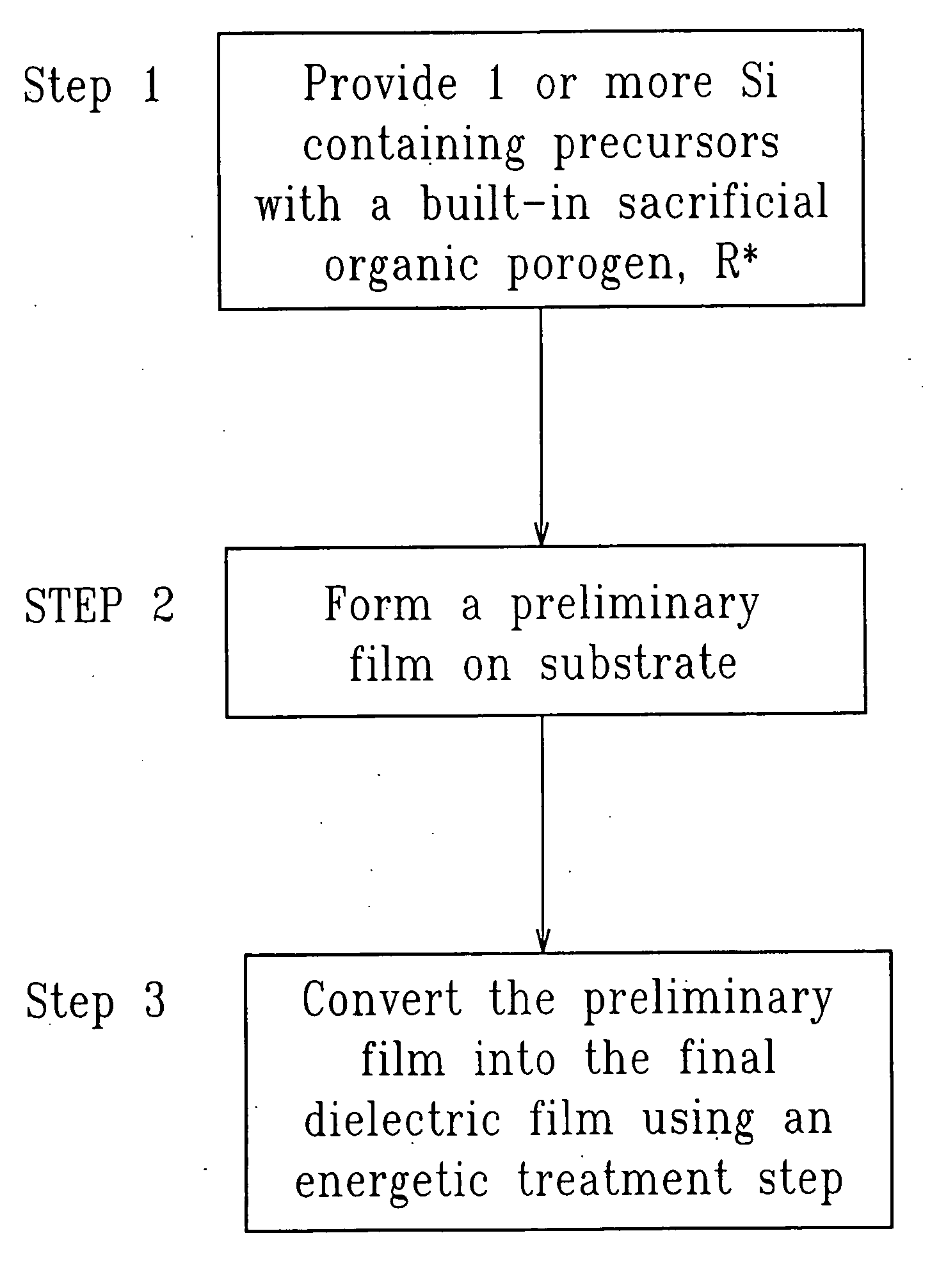
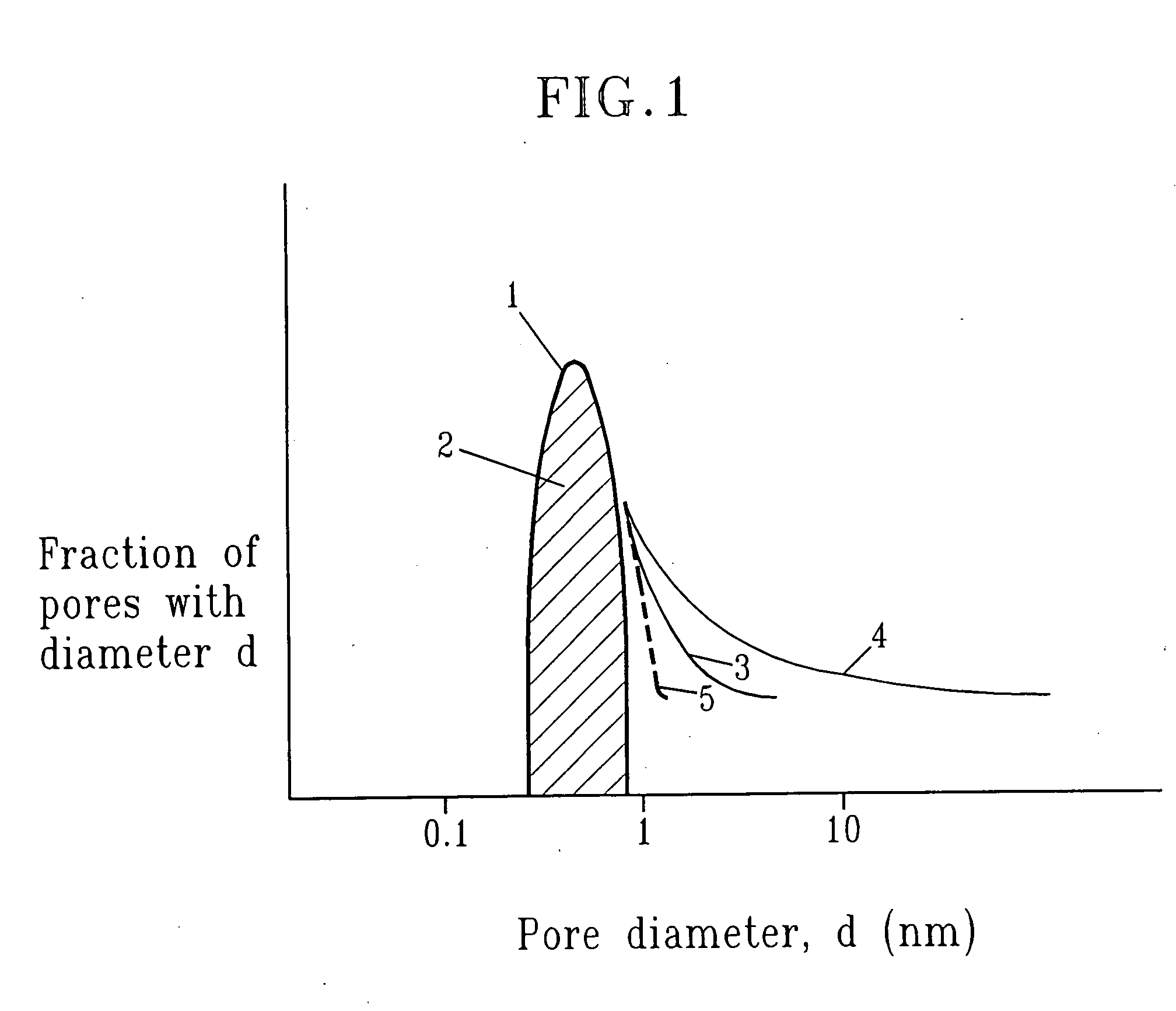
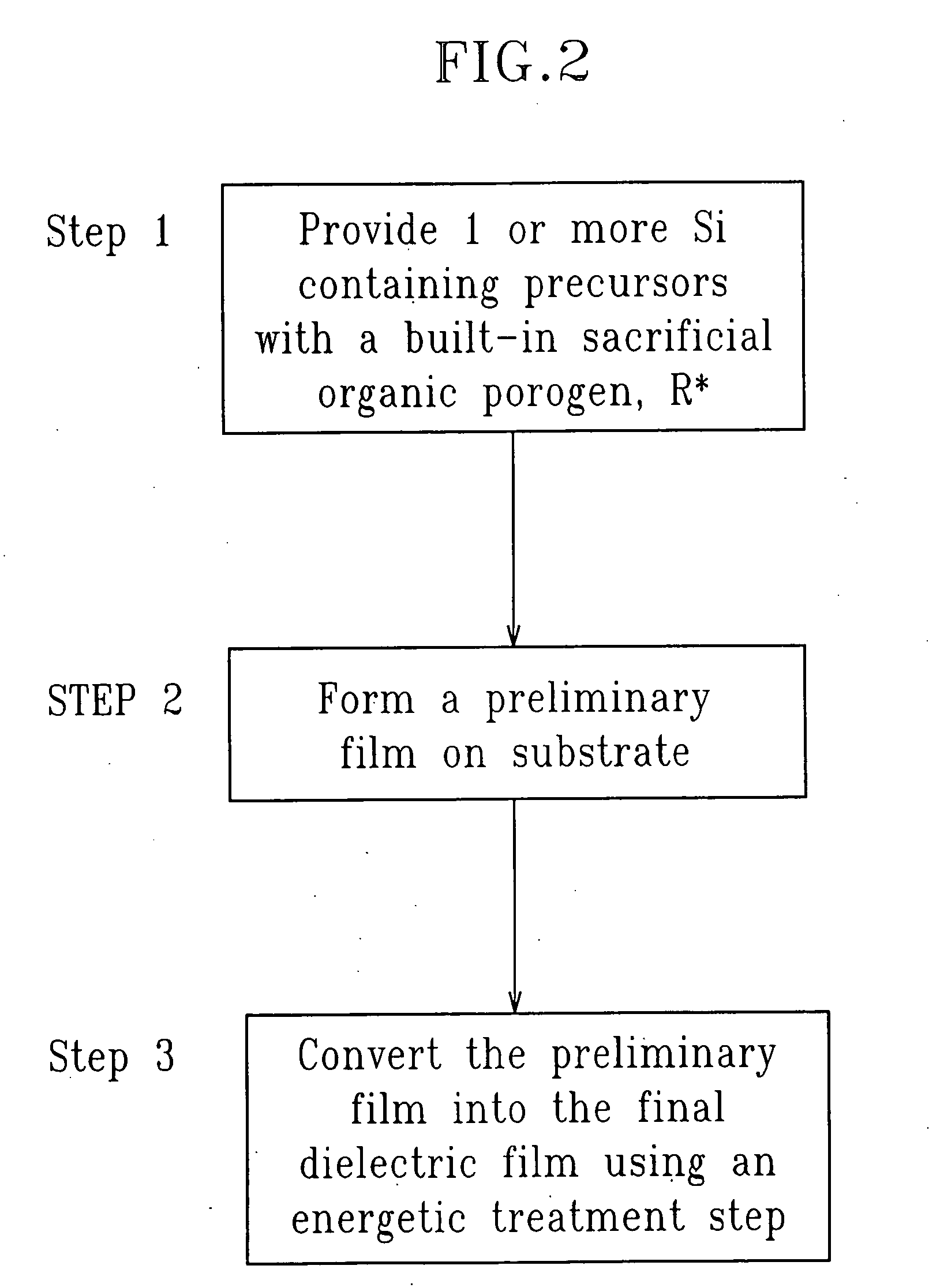
SiCOH film preparation using precursors with built-in porogen functionality
a technology of porogen and precursor, which is applied in the direction of liquid surface applicators, coatings, chemical vapor deposition coatings, etc., can solve the problems of increasing signal delays in ulsi electronic devices, affecting the production efficiency of sicoh dielectrics, and not being thermally stable, etc., to achieve better mechanical properties, improve film properties, and improve the effect of porosity control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
[0059] Referring to Scheme 1, R═alkyl, R1 and R2 may be alkyl, aryl, or alkoxy, R3 may be hydrogen, alkyl, aryl, cycloalkyl or alkoxy and R4 may be alkyl, aryl or alkoxy. Moreover, the reactions are shown to provide a route to polymerizable precursors where the silicon substituents are separated by a two-carbon bridge (Si—CH2—CH2—Si bonding unit). At the top of Scheme 1 are example starting materials within the These molecules are substituted derivatives of disilane. Shown at the top of Scheme 1 are highly substituted disilane derivatives, but related molecules with fewer alkoxy (OR) substituents may be used within the invention. Fluorine and chlorine substituents are also acceptable for the subsequent oxidative addition. The starting material may be symmetrical or unsymmetrical. A preferred substituent is alkoxy and most preferably multiple alkoxy substituents as shown at the top of Scheme 1 are used. The disilane bond is prone to oxidative cleavage and addition to Pi bonds in the...
second embodiment
[0063] Within the second embodiment, the precursors (31) and (33) are converted to a cyclic precursor or a prepolymer prior to forming the preliminary film. Referring now to Scheme 2, precursor (31) wherein at least one of R1 or R2 is R*, preferably an alkoxy, further transformed by acid or base-catalyzed hydrolysis. Typically, the starting material is dissolved in methylene chloride and treated with 0.1N aqueous HCl solution at 25° C. for 24 hrs. The volatiles are removed and the residue redissolved in methylene chloride and dried over 4 Å molecular sieves. Higher dilutions favor the formation of cyclics when only two alkoxy substituents are present in the starting materials (K. Rahimian et al Chem. Mater. 2005, 17, 1529).
[0064] Depending on the stereochemistry of the oxidative addition, reaction with one equivalent of water can produce 2-oxa-1,3-disilylcyclopentanes containing additional finctionality for subsequent condensation, as shown in Scheme 2. Path A, producing precursor...
third embodiment
[0066] Referring now to Scheme 3, a third embodiment within the invention is shown in which a related synthetic procedure is applied to norbomene substituted monomers. This embodiment builds a Si—C copolymer that contains subunits related to a successful organic porogen molecule bicycloheptadiene (norbomadiene). First precursor (39) is selected, and the preliminary film contains the structure shown schematically as (41) formed by hydrolysis and condensation and is coated on the substrate. In step (42), controlled heating causes the loss of the polycyclic hydrocarbon fragments by retro Diels Alder reaction, forming a network organosilane containing bridging ethylene segments (43), a further stage of the preliminary film.
[0067] The steps of FIG. 2 which shows hydrolysis steps; in the above, the hydrolysis / condensation occurs in 3A, are applied. The double bonds in (43) are further incorporated into the final film and converted to single bonds during the energetic treatment step with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dielectric constant | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com