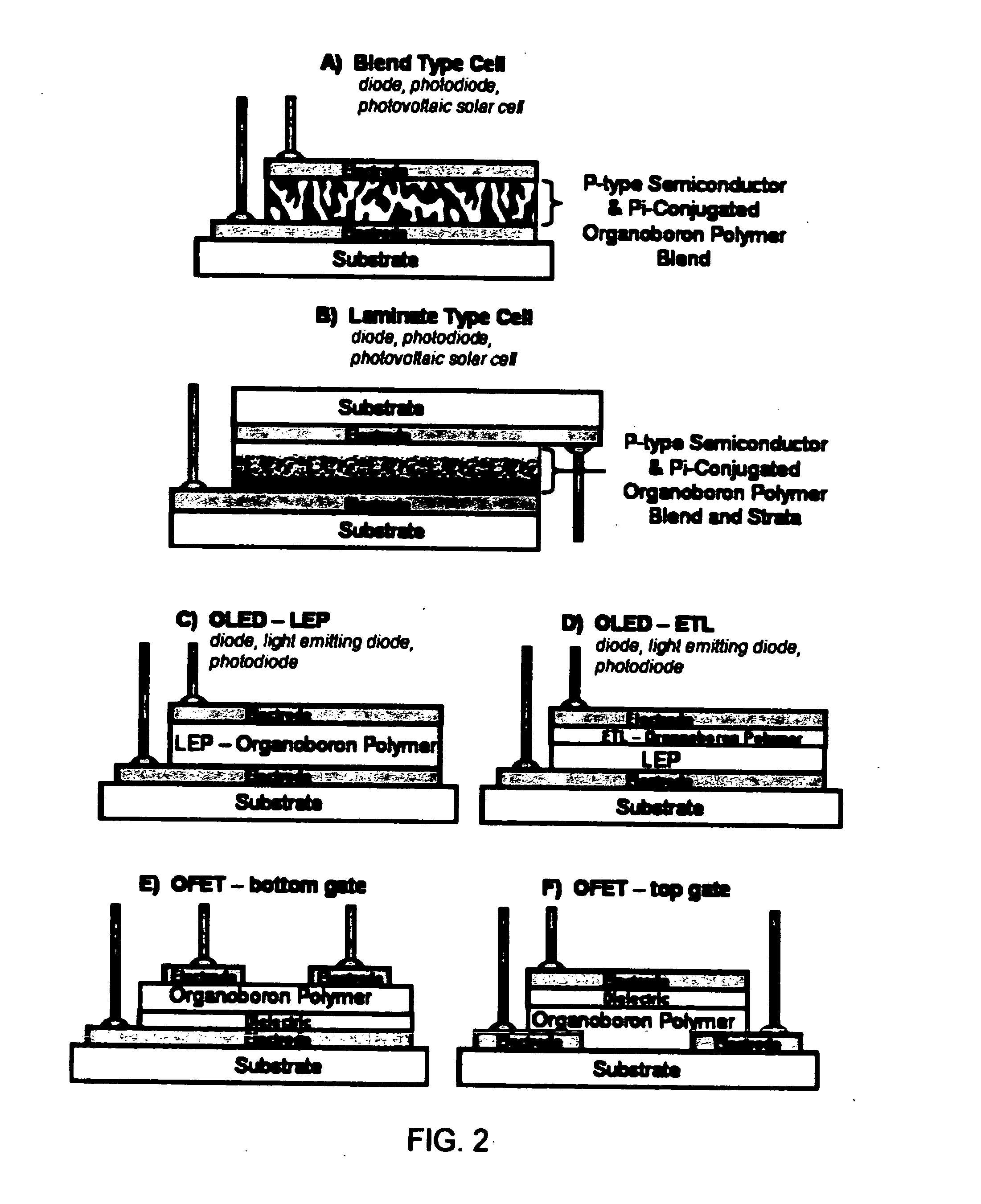
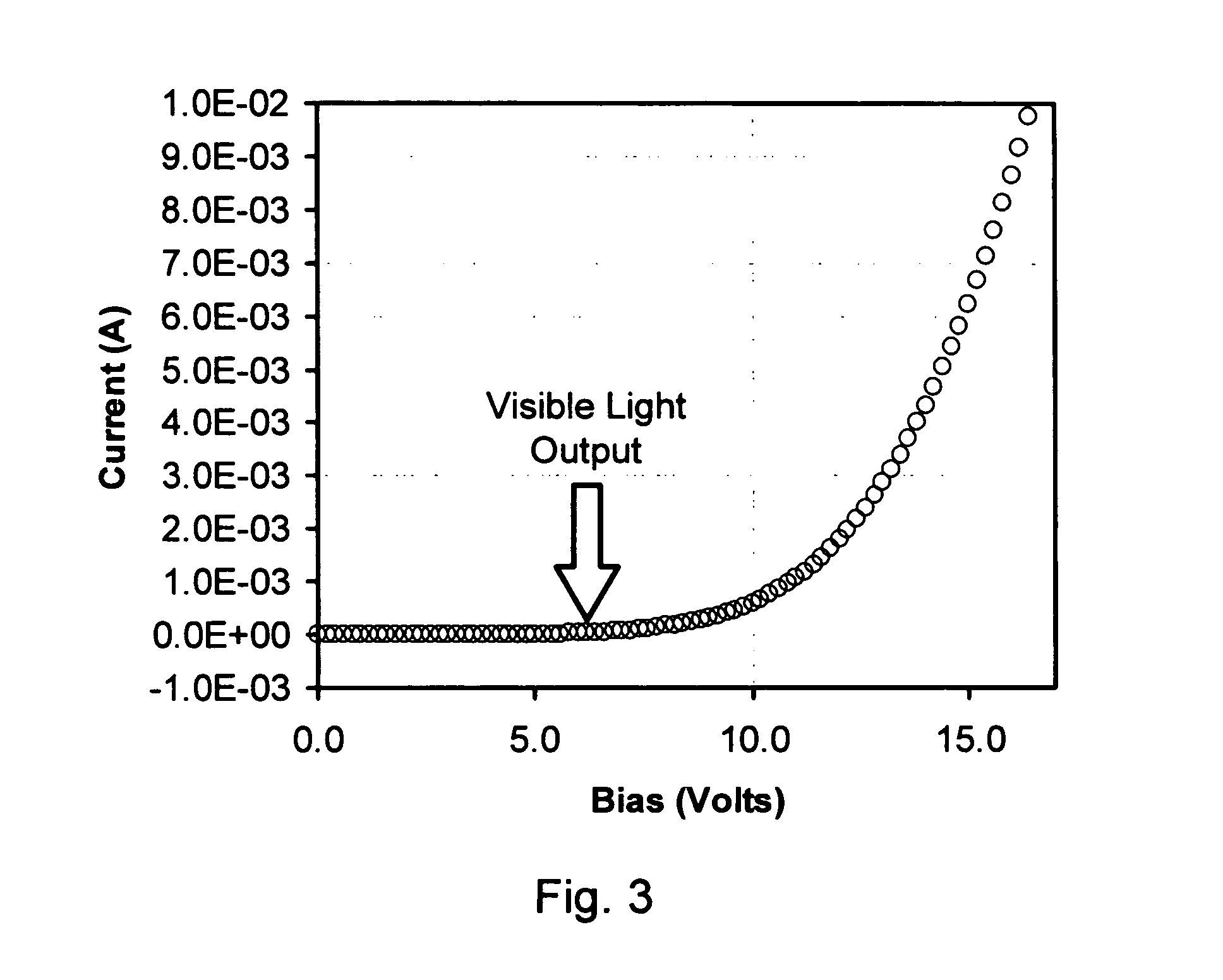
Use of pi-conjugated organoboron polymers in thin-film organic polymer electronic devices
a technology of organoboron and organoboron polymer, which is applied in the direction of solid-state devices, discharge tubes/lamp details, natural mineral layered products, etc., can solve the problems of difficult design of electron-deficient conjugated polymer systems, poor properties of current n-type semi-conducting polymers, and unstable other icps in their semi-conducting state, etc., to reduce the electron-deficiency of polymers, improve the properties, and the aromatic units
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Poly(arylboranes)
[0104] Poly(arylborane)s were prepared in a two-step synthesis from commercially available starting materials according as illustrated in Scheme 3; first two different borane reagents were prepared with a mesityl or tripyl group (step A), then the borane reagent was polymerized with an aromatic dibromo compound after transforming it into its Grignard reagent (step B). Representative syntheses are given below.
[0105] Scheme 4 shows the structure of the polymers prepared in this way, six with mesityl substituents on the boron atoms (x series) and seven with tripyl substituents (y series). Seven different dibromo-aromatic compounds were used as the starting materials: 5,5′-dibromobithiophene (polymers 3a), 2,5-dibromo-3-hexyl-thiophene (polymers 3b), 1,4-dibromo-2,5-bis(decyloxy)benzene (polymers 3c), 9,9-dihexyl-2,7-dibromofluorene (polymers 3d), 9,9-didodecyl-2,7-dibromofluorene (polymers 3e), 2,5-dibromo-3-dodecyl-thiophene (polymers 3f), and 9,9-diiso...
example 2
Synthesis of Poly(vinylborane)s
[0114] Poly(vinylborane)s were synthesized by reacting trans-1,2-bis(tributyltin)ethene with dichloroboranes (Scheme 5). 1H-NMR and elemental analysis indicated that the desired products were formed.
Synthesis of trans-1,2-bis(tributyltin)ethene
[0115] Tribuytyltin hydride (3.15 g, 10.8 mmol) and ethynyltributylstannane (3.4 g 10.8 mmol) and a catalytic amount of azobisisobutyronitrile (AIBN) were heated at 90° C. for 10 hours under an inert argon atmosphere. The reaction was vacuum distilled and the product was obtained in 95% yield.
Synthesis of Dichlorohexylborane
[0116] A solution of 1.91 g (16.25 mmol) of boron trichloride and 1.37 g (2.03 mL, 16.25 mmol) of 1-hexene in 5 cm3 of pentane was stirred under argon at −78° C. for 15 minutes. Tributylsilane (3.26 g, 16.25 mmol) was added dropwise to the stirred reaction mixture and left to stir overnight at room temperature. Vacuum distillation yielded dichlorohexylborane in 90% yield.
Synthesis of 3i...
example 3
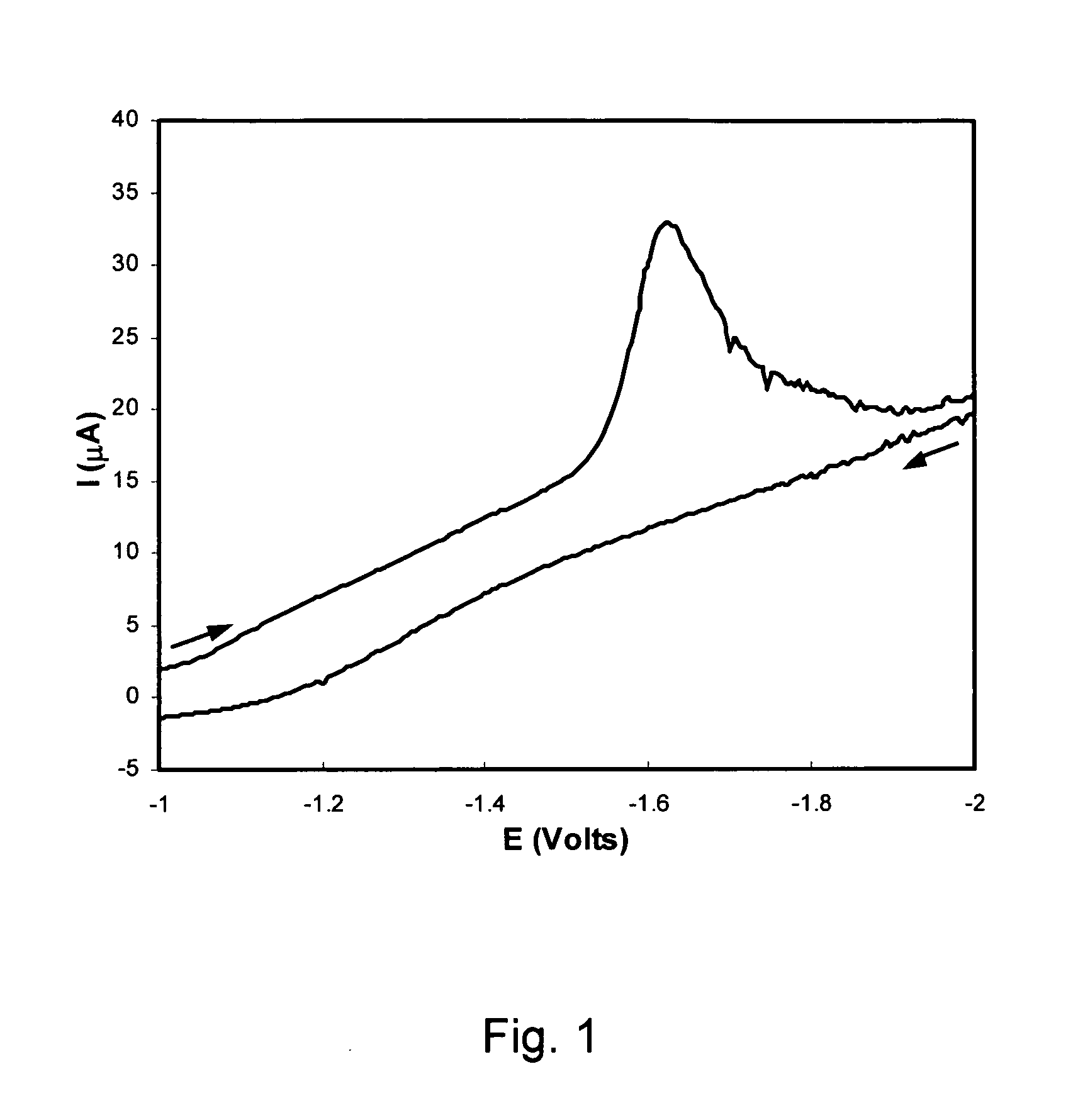
[0118] To identify the n-type semiconducting behavior of these polymers, we cast thin films onto a platinum working electrode and subjected these films to cyclic voltammetry. FIG. 1 shows the reduction of the 3ay polymer under an argon atmosphere. N-type behavior is generally indicated by a facile reduction process (electron injection) of the film on the electrode surface. We observe a reduction process in our materials, however, this reduced or n-doped form of the material is unstable or highly soluble because no film remains on the electrode after reducing the film.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Dielectric polarization enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com