Process for converting hydrocarbon feedstocks with electrolytic recovery of halogen
a technology of electrolysis recovery and hydrocarbon feedstock, which is applied in the direction of solid-state devices, combustible gas purification/modification, non-metal raffination, etc., can solve the problems of difficult separation of intermediates, ineconomic value, and hbr electrolysis route to hydrogen, and achieves the effect of higher efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Bromination of Methane
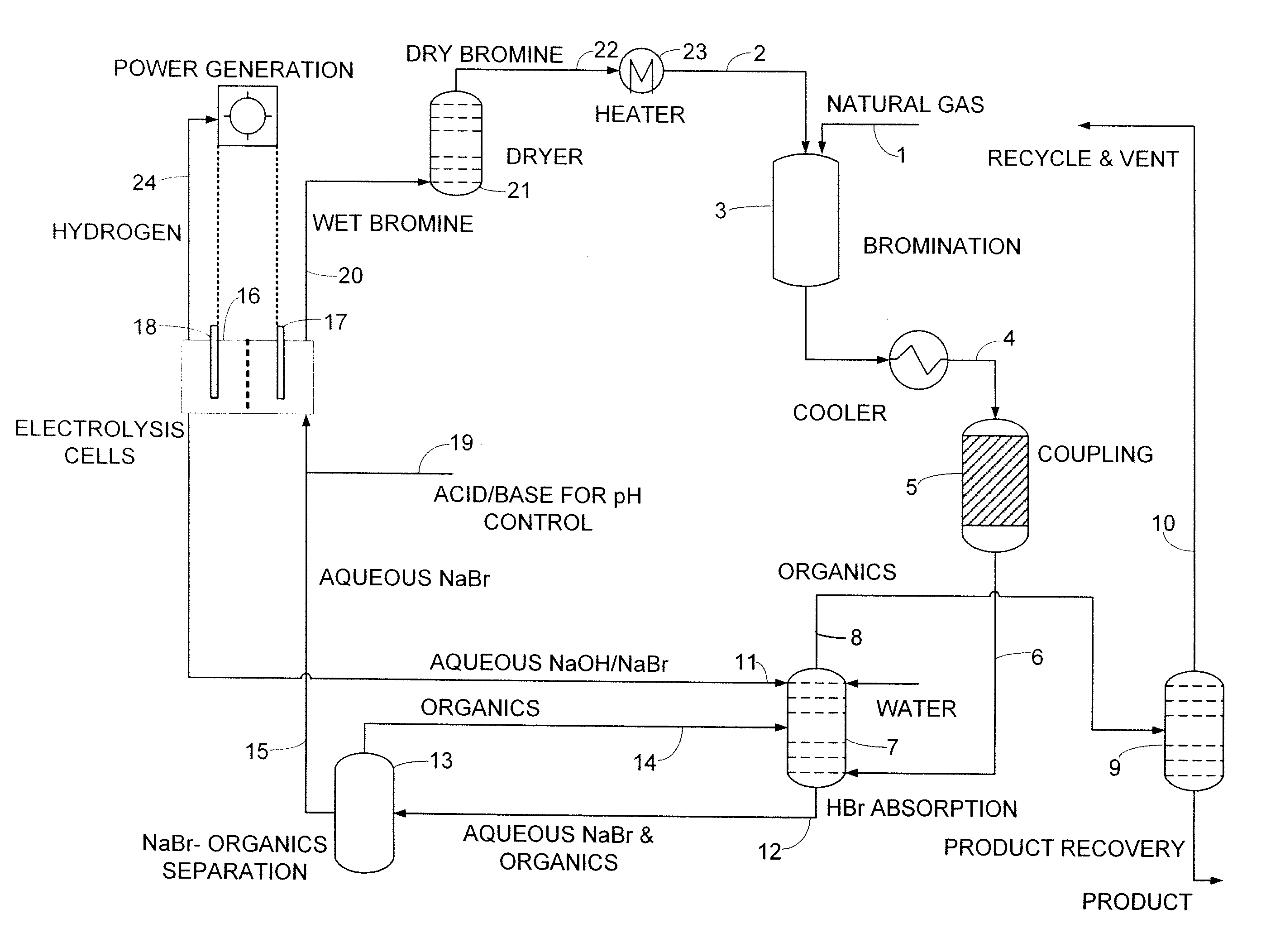
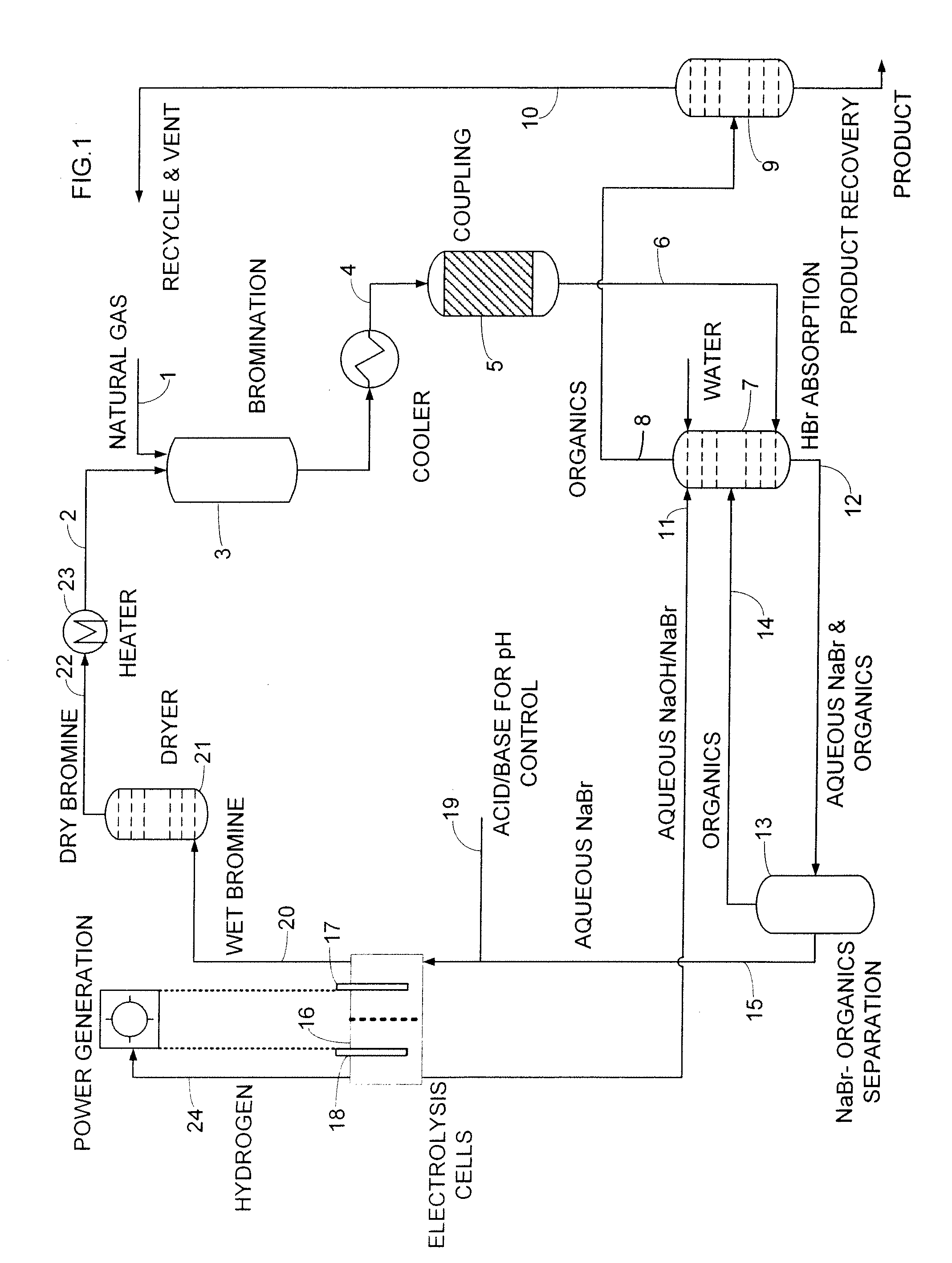
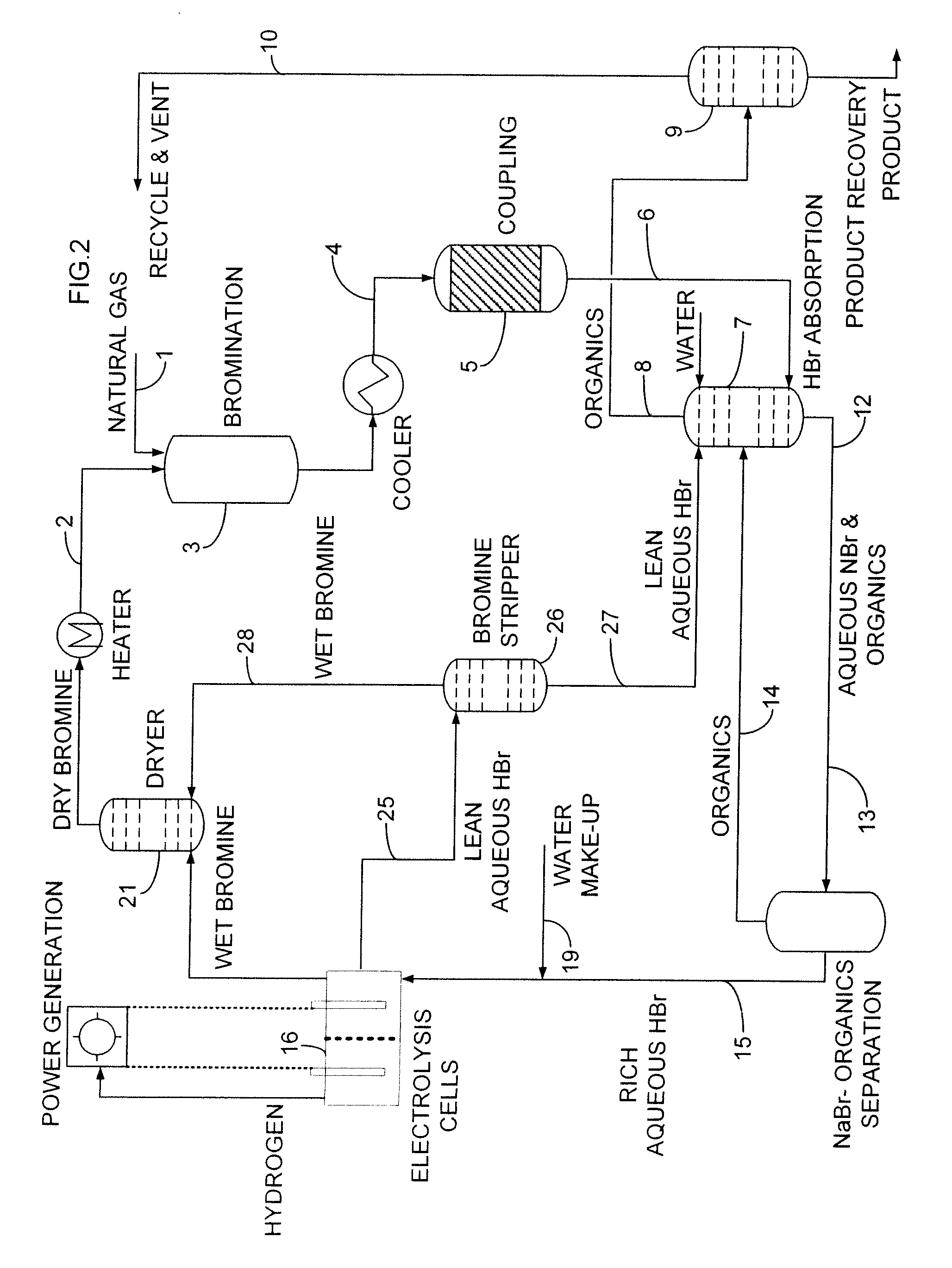
[0091]Methane (11 sccm, 1.0 atm) was combined with nitrogen (15 sccm, 1.0 atm) at room temperature via a mixing tee and passed through an 18° C. bubbler full of bromine. The CH4 / N2 / Br2 mixture was passed into a preheated glass tube (inside diameter 2.29 cm, length, 30.48 cm, filled with glass beads) at 500° C., where bromination of methane took place with a residence time of 60 seconds, producing primarily bromomethane, dibromomethane and HBr:
CH4+Br2→CH3Br+CH2Br2+HBr
[0092]As products left the reactor, they were collected by a series of traps containing 4M NaOH, which neutralized the HBr and hexadecane (containing octadecane as an internal standard) to dissolve as much of the hydrocarbon products as possible. Volatile components like methane were collected in a gas bag after the HBr / hydrocarbon traps.
[0093]After the bromination reaction, the coke or carbonaceous deposits were burned off in a flow of heated air (5 sccm) at 500° C. for 4 hours, and the CO2 was cap...
example 2
CH3Br Coupling to Light Olefins
[0094]2.27 g of a 5% Mg-doped ZSM-5 (CBV8014) zeolite was loaded in a tubular quartz reactor (1.0 cm ID), which was preheated to 400° C. before the reaction. CH3Br, diluted by N2, was pumped into the reactor at a flow rate of 24 μl / min for CH3Br, controlled by a micro liquid pump, and 93.3 ml / min for N2. The CH3Br coupling reaction took place over the catalyst bed with a residence time of 0.5 sec and a CH3Br partial pressure of 0.1 based on this flow rate setting.
[0095]After one hour of reaction, the products left the reactor and were collected by a series of traps containing 4M NaOH, which neutralized the HBr and hexadecane (containing octadecane as an internal standard) to dissolve as much of the hydrocarbon products as possible. Volatile components like methane and light olefins were collected in a gas bag after the HBr / hydrocarbon traps.
[0096]After the coupling reaction, the coke or carbonaceous deposits were burned off in a flow of heated air (5 s...
example 3
CH3Br Coupling to BTX
[0098]Pellets of Mn ion exchanged ZSM-5 zeolite (CBV3024, 6 cm in length) were loaded in a tubular quartz reactor (ID, 1.0 cm), which was preheated to 425° C. before the reaction. CH3Br, diluted by N2, was pumped into the reactor at a flow rate of 18 μl / min for CH3Br, controlled by a micro liquid pump, and 7.8 ml / min for N2. The CH3Br coupling reaction took place over the catalyst bed with a residence time of 5.0 sec and a CH3Br partial pressure of 0.5 based on this flow rate setting.
[0099]After one hour of reaction, the products left the reactor and were collected by a series of traps containing 4M NaOH, which neutralized the HBr and hexadecane (containing octadecane as an internal standard) to dissolve as much of the hydrocarbon products as possible. Volatile components like methane and light olefins were collected in a gas bag after the HBr / hydrocarbon traps.
[0100]After the coupling reaction, the coke or carbonaceous deposits were burned off in a flow of heat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical power | aaaaa | aaaaa |
| alkaline | aaaaa | aaaaa |
| electrical energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com