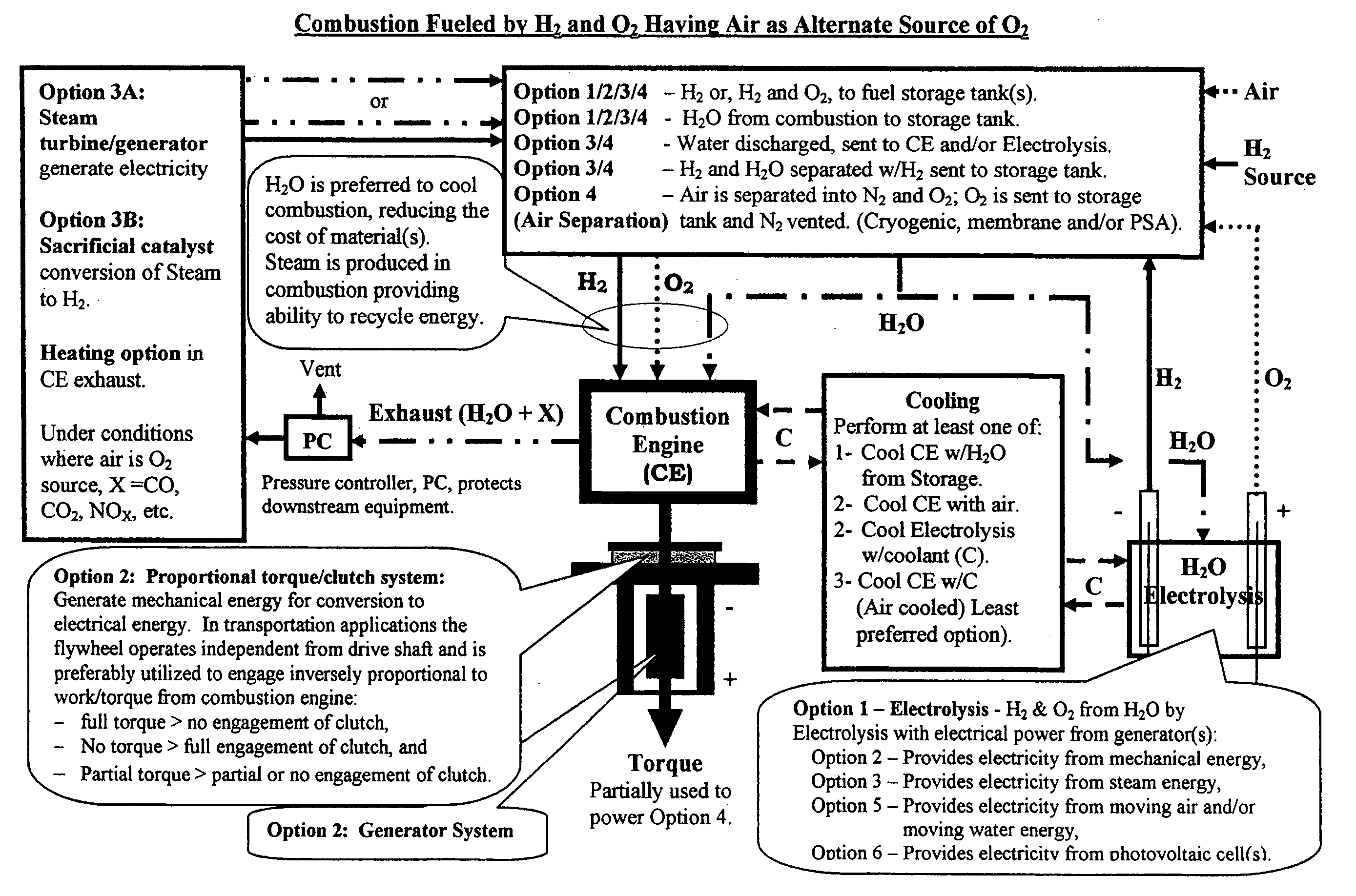
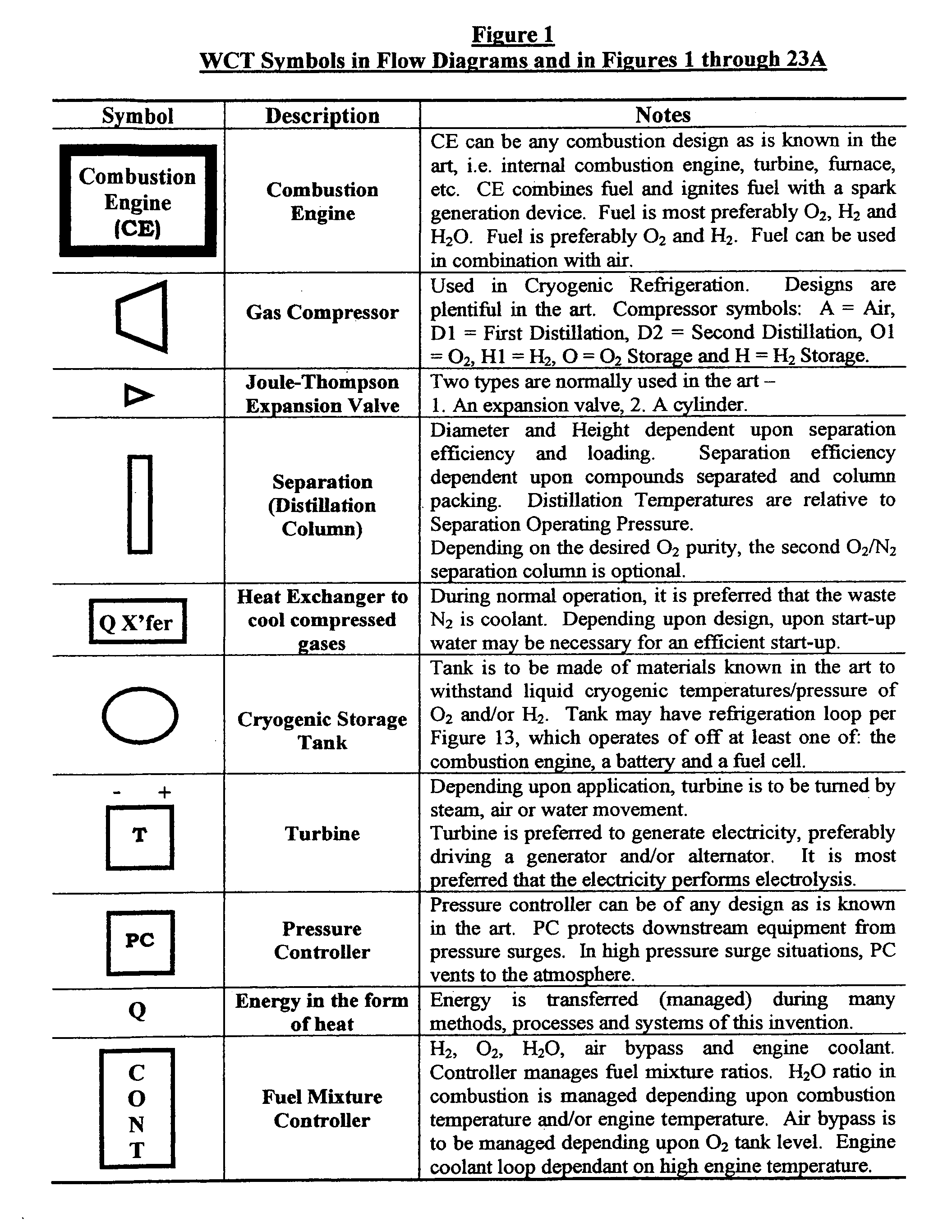
Water combustion technology- methods, processes, systems and apparatus for the combustion of hydrogen and oxygen
a technology of water combustion and hydrogen, applied in the direction of liquefaction, machines/engines, electric generator control, etc., can solve the problems of inability of plant life to convert enough of manmade cosub>2 /sub>2 into osub>2, inability of fossil fuel combustion systems to manage temperature without significant entropy, and inability to convert co, an incomplete combustion by-product, to osub>2, etc., to achieve the effect of osub
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
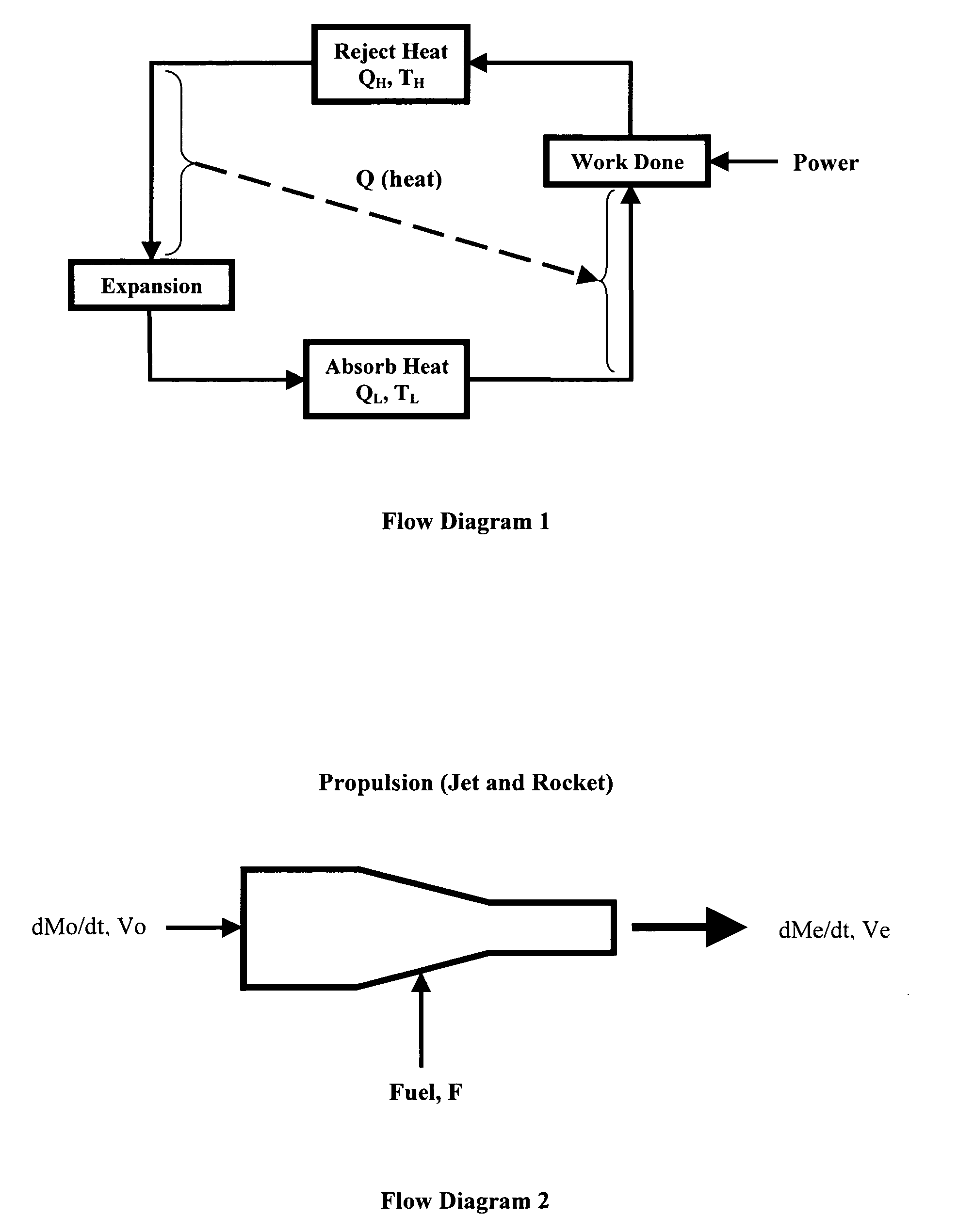
[0149]A traditional gasoline internal combustion engine obtains approximately 20 miles per gallon. Performing an energy balance on the engine, according to FIG. 2:
EF=EW+EEX+EC+Efric+CE
EF=20 mpg+≈35% EF+≈35% EF+≈9% EF+≈1% EF
EFEW+≈80% EF in energy losses for internal CE(s).
EF=20 mpg+80% EF; EF=100 mpg and EW≈20% EF
Again,
[0150]
EF=EW+EEX+EC+Efric+CE
[0151]Assuming: 1) complete engine insulation, 2) a steam turbine with 80% efficiency, 2) a generator with 90% efficiency and 3) an electrolysis unit with 80% efficiency turns EX and EC together into approximately 30% EF
[0152]Using WCT,
EF=EW+30% EF+≈9% EF+≈1% EF
EF=EW+≈40% EF (energy losses); EW (WCT)=60% EF
example 2
[0153]Referencing CRC Handbook of Chemistry and Physics, the total available combustion energy for n-Octane is approximately 1,300 kcal / mole; at 114 lb / lb mole EF=11.4 kcal / g and at 454 g / lb. EF=5176 kcal / lb. (This excludes endothermic losses in the formation of NOX.) Further, the density of n-Octane is approximately 5.9 lb / gallon, which leads to energy figures for n-Octane in the average automobile:
EF≈100 mpg=17 mile / lb.=5176 kcal / b.; EW≈20 mpg=3.4 mile / lb.=1143 kcal / lb.
The total available energy for the combustion of hydrogen is 68 kcal / mole; at 2 lb / lb mole EF=34 kcal / g=15436 kcal / lb. Therefore, on a mass basis, H2=34 / 11.4≈3 times more energy per pound.
[0154]Using WCT, 60% / 20%=3 times more efficient. Correlating, energy figures for WCT in the average automobile:
[0155]First, the fuel availability must be calculated. H2 is 100% as delivered. Since cryogenics are at least approximately 16% efficient, the production of O2 is conservatively estimated to be 16% efficient.
2 / 3×1+1 / 3×0.16...
example 3
[0157]According to the Chemical Market Reporter, H2 has a market price of approximately $0.50 / lb. and gasoline has a price of approximately $1.60 per gallon or approximately $0.27 per pound. Utilizing traditional hydrocarbon combustion technology in transportation, the cost per mile for fuel is:
$0.27 per lb. / 3.4 mile per lb.=$0.08 per mile for gasoline
[0158]Utilizing the WCT with $0.50 / lb. H2, the cost per mile for fuel is:
$0.50 per lb. / 21.3 mile per lb.=$0.023 per mile
[0159](This calculation can be altered to the current market price of hydrogen.)
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| electrical energy | aaaaa | aaaaa |
| mechanical energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com