Method for producing silver nanoparticles and conductive ink
a silver nanoparticle and conductive ink technology, applied in the field of producing metal nanoparticles, can solve the problems of limited mass production efficiency, low yield rate of metal nanoparticles by this existing method, and limitation of metal nanoparticles' yield rate, etc., to achieve high yield rate, simple process, and uniform size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
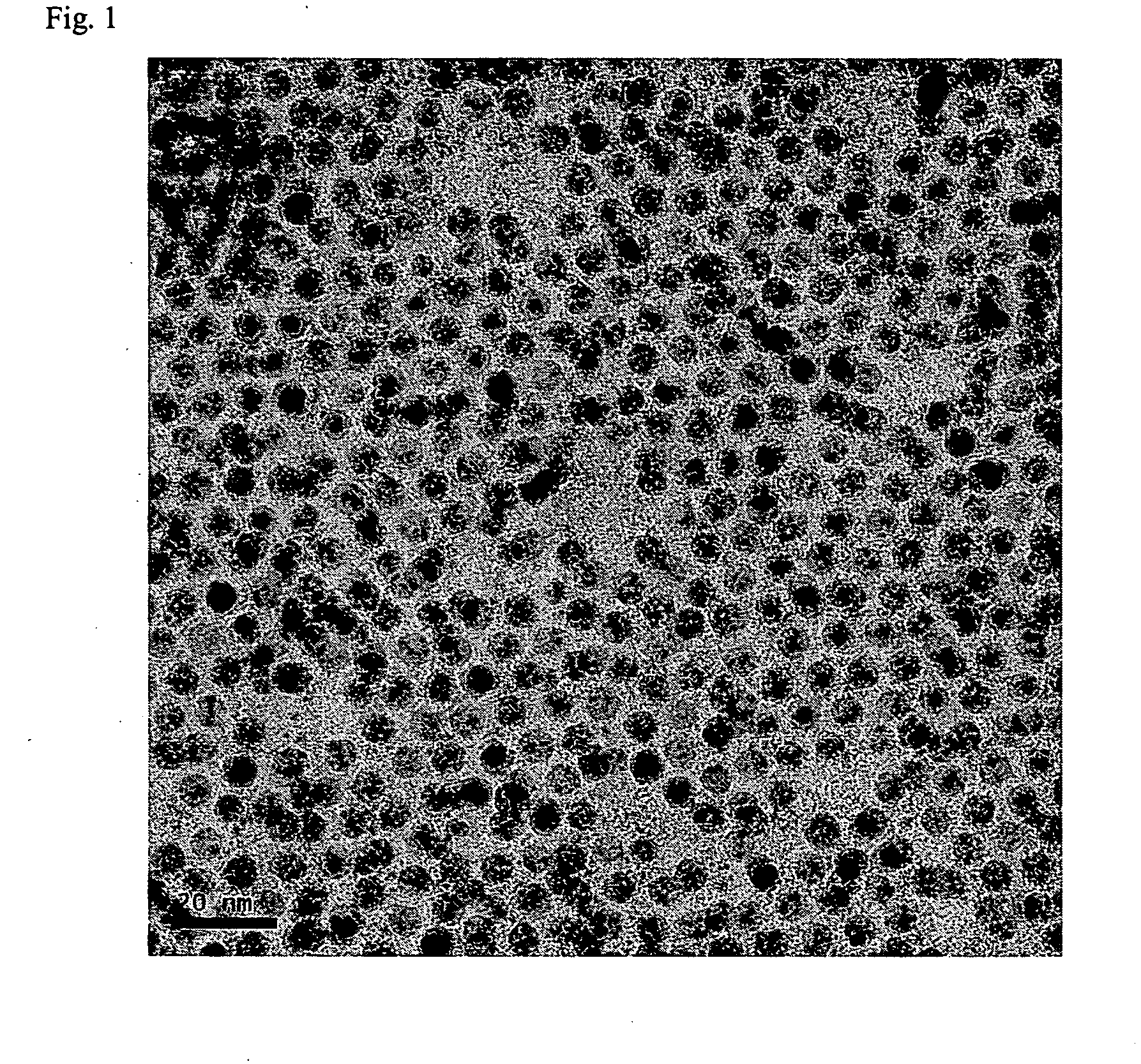
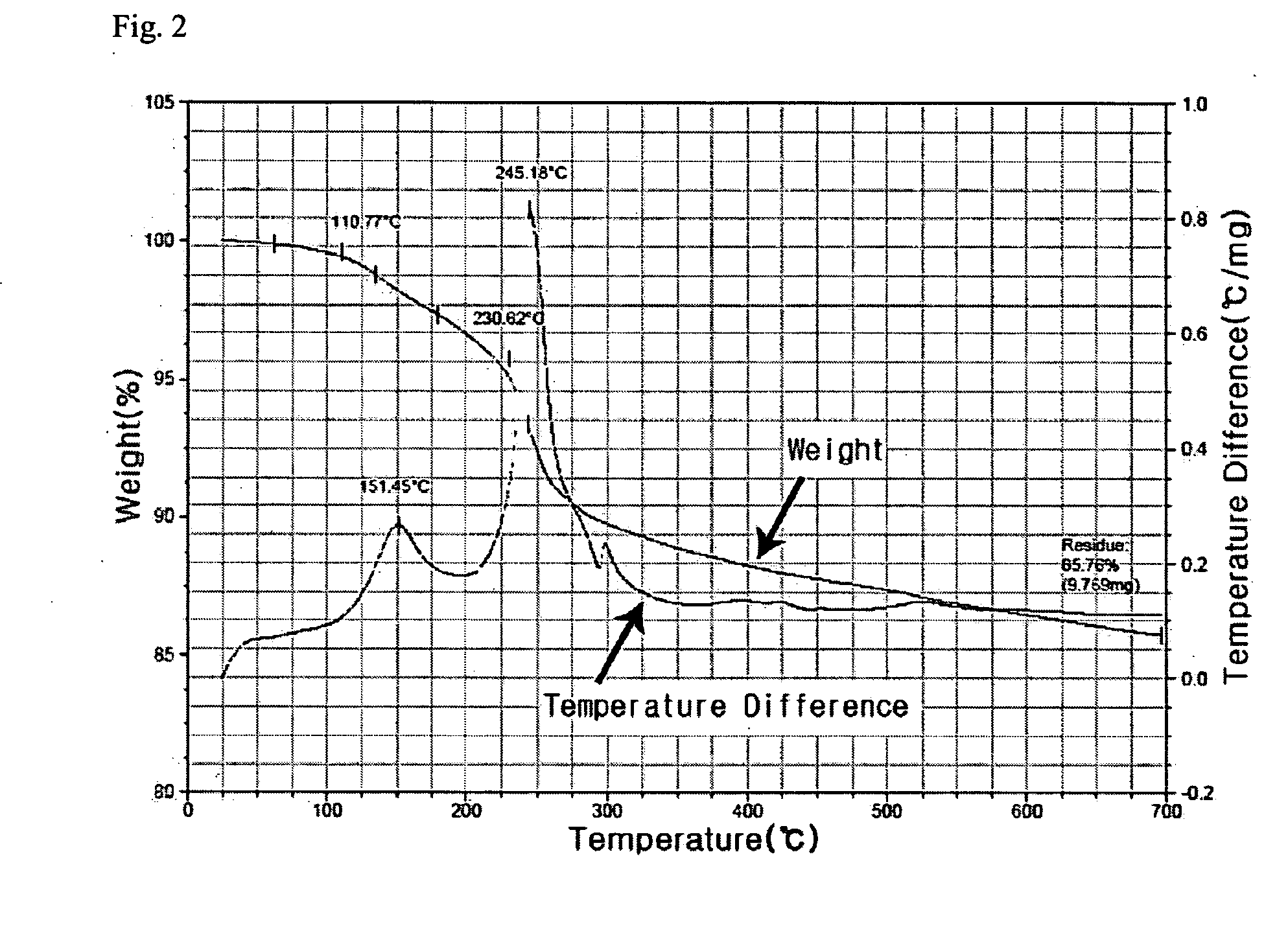
[0046]170 g of AgNO3 and 20 g of copper (II) acetoacetate (Cu(acac)2) compound were mixed to 300 g of toluene solvent and then 100 g of butylamine was further added and stirred. 50 g of palmitic acid was added to this mixed solution. The reaction mixture was heated to 110° C. and then sustained by stirring for 2 hours and cooled to room temperature (28° C.). Ag nanoparticles thus formed were added to methanol and centrifuged to precipitate and the precipitates, Ag nanoparticles, were separated out. As shown in FIG. 1, 90 g of metal nanoparticles that have uniform size distribution of 4 nm were obtained. As illustrated in FIG. 2, the result of TGA analysis shows that Ag content by percentage is 85 wt %. When Ag nanoparticles thus retrieved were re-dispersed in an organic solvent, high dispersion stability was observed, re-dispersion yield rate was also high.
example 2
[0047]170 g of AgNO3 and 20 g of copper (II) acetoacetate (Cu(acac)2) compound were mixed to 300 g of toluene solvent and then 100 g of butylamine was further added and stirred. 50 g of oleylamine was added to this mixed solution, and these mixed compounds were heated to 110° C. and then sustained by stirring for 1 hour and cooled to room temperature (28° C.). Ag nanoparticles thus formed were added to methanol and centrifuged to precipitate and the precipitates, Ag nanoparticles, were separated out. As shown in FIG. 3, 90 g of metal nanoparticles that have uniform size distribution of 5 nm were obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com