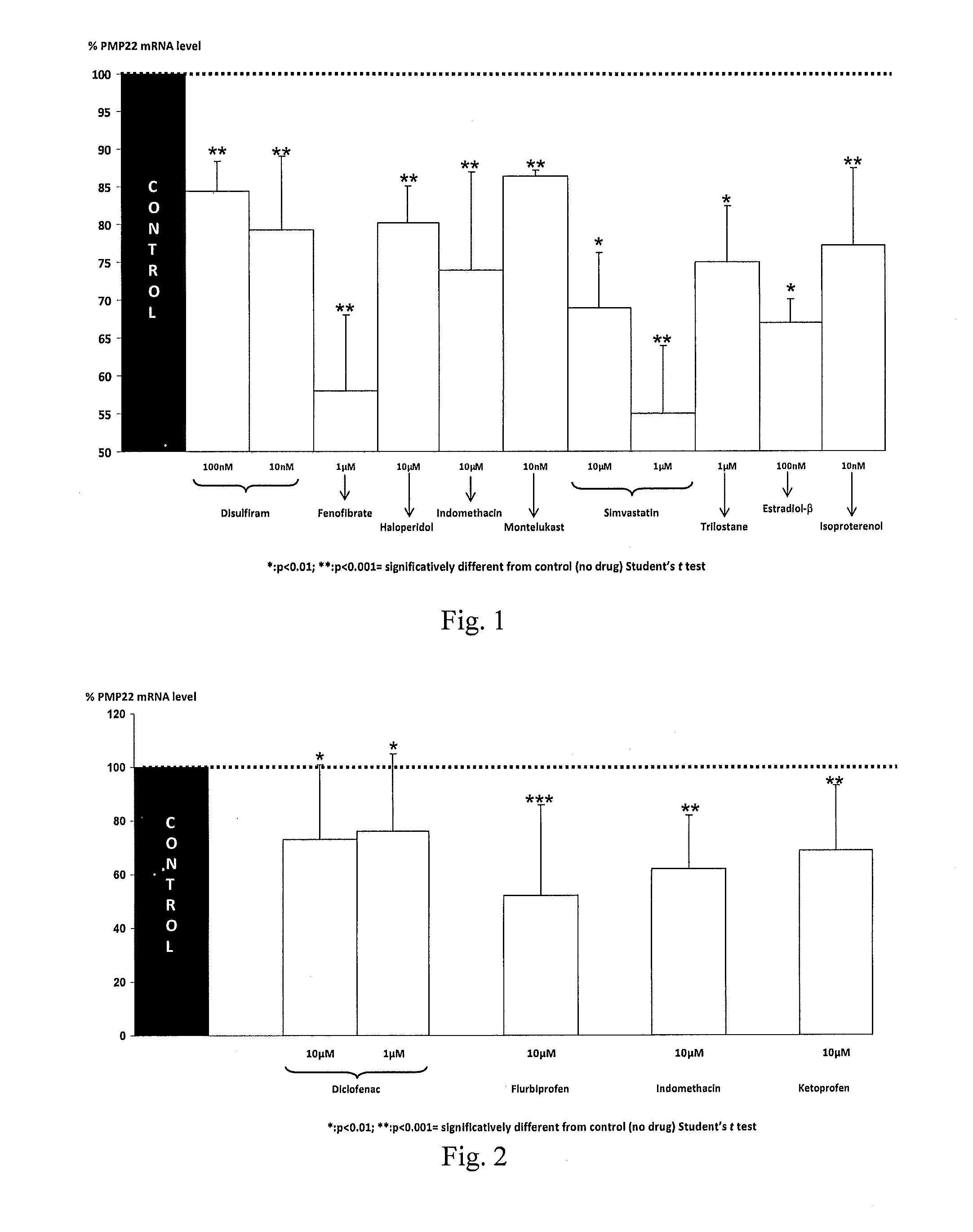
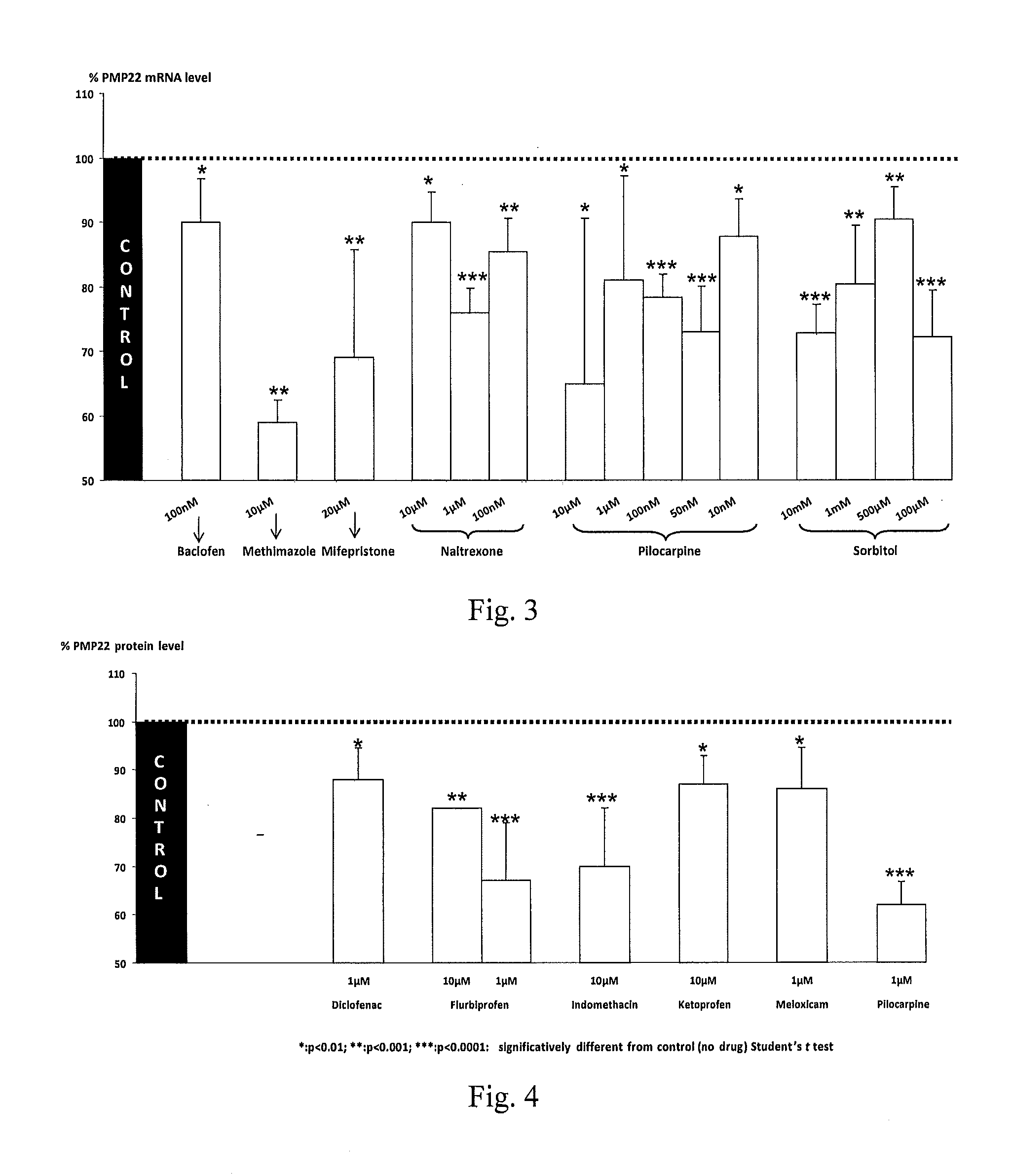
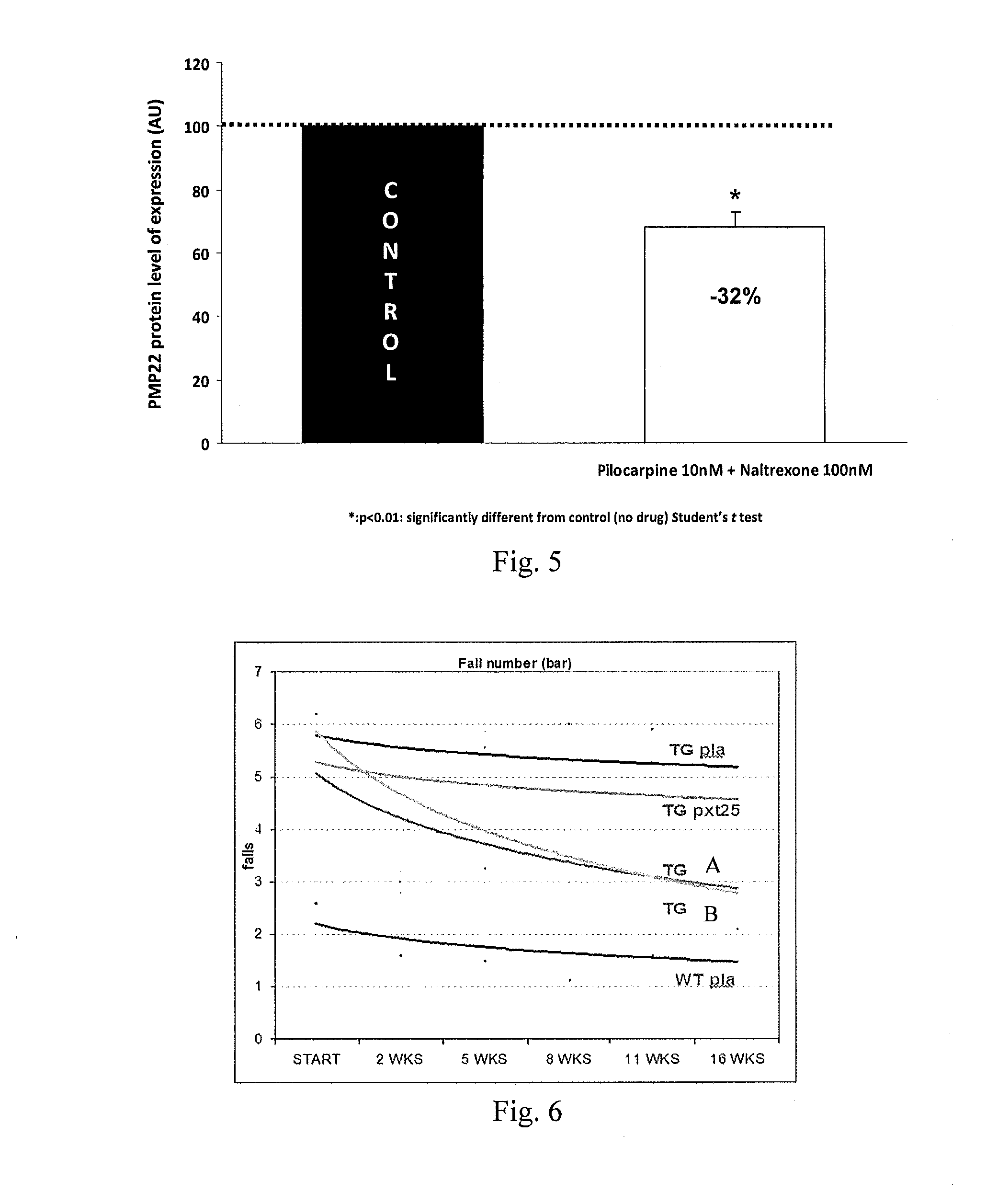
Therapeutic approaches for treating cmt and related disorders
a technology of cmt and related disorders, applied in the field of treatment of charcotmarietooth disease and related disorders, can solve problems such as invalidation of diseases, and achieve the effect of reducing pmp22 expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
1. Cell Culture
[0196]1.1: Commercially Available Rat Primary Schwann Cells
[0197]Vials of rat Schwann cells (SC) primary culture (Sciencell #R1700) are defrost and seeded at the density of 10,000 cells / cm2 in “Sciencell Schwann cell medium” (basal medium from Sciencell #R1701) in poly-L-lysine pre-coated 75 cm2 flasks. The culture medium is composed of basal medium, 5% Fetal Bovine Serum (3H-Biomedical AB #1701-0025), 1% Schwann cell growth supplement (3H Biomedical AB #1701-1752), 1% Gentamicin (Sigma #G1397) and 10 μM of Forskolin (Sigma #F6886) to promote their proliferation.
[0198]After reaching confluency (4 to 10 days depending on cell batch), Schwann cells are purified by gentle agitation or by thy1.1 immunopanning that allow SC isolation from adherent fibroblasts, to produce cultures that are at least 95% pure. SC are then counted (Tryptan blue method) and seeded in poly-L-lysine pre-coated 75 cm2 flask in the same SC medium. At confluency, cells are rinsed, trypsinized (tryps...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| final volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com