Tissue scaffolds for electrically excitable cells
a tissue scaffold and excitable cell technology, applied in the field of tissue scaffolds, can solve the problems of limited predictive power of human clinical trials and post-market surveillance of preclinical data acquired through the use of rodent brain slices, high animal care and labor-intensive use costs, etc., and achieves improved predictive power, reduced cost, and the effect of improving the quality of li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Electrospinning of Random-Nonwoven and Aligned Photoreactive Nanofibers
[0156]Photoreactive polycaprolactone nanofibers were prepared by electrospinning solutions containing 10% poly(ε-caprolactone) (PCL, with an average molecular weight of 80 kDa, purchased from Sigma-Aldrich) and 0.1% Photoreactive Crosslinker in a 1:1 solution of tetrahydrofuran:N,N-dimethylformamide. Electrospinning procedure was as described in Example 2 of U.S. Patent Application Publication No. US 2014 / 0294783 A1 (Jie Wen et al., published 2 Oct. 2014). Random, nonwoven nanofiber mats (meshes) were collected as described in the referenced Example.
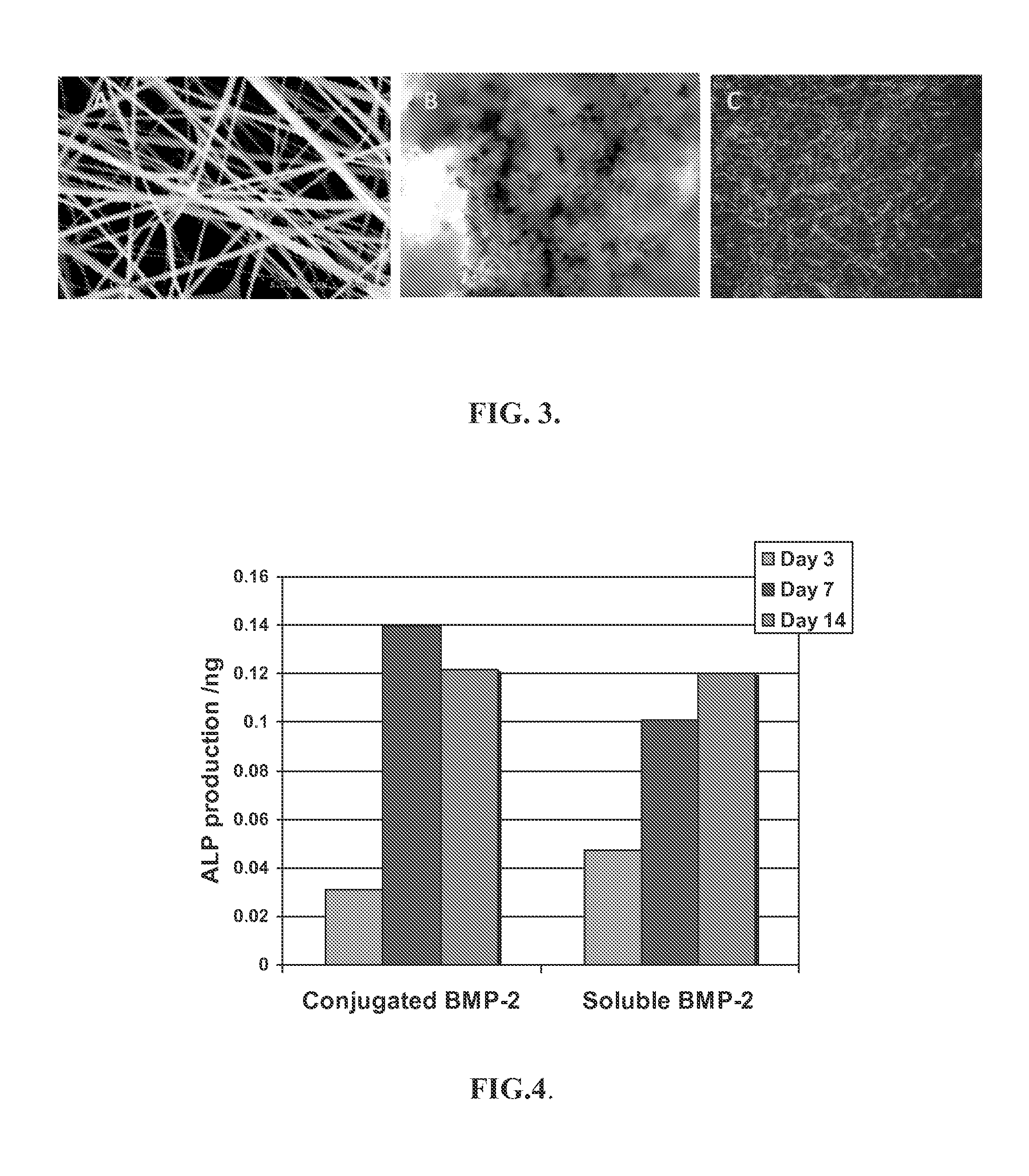
[0157]The formed nanofiber mats were removed, placed in a vacuum chamber for at least 48 hours to remove organic solvent residue, then stored in a desiccator. Fiber diameter, morphology and pore size of the dried nanofibers were characterized using light and scanning electron microscopy (SEM). FIG. 3A illustrates an SEM image of PCL nanofibers containing 1% Photoreact...
example 2
Photopatterning of Nanofiber Mats
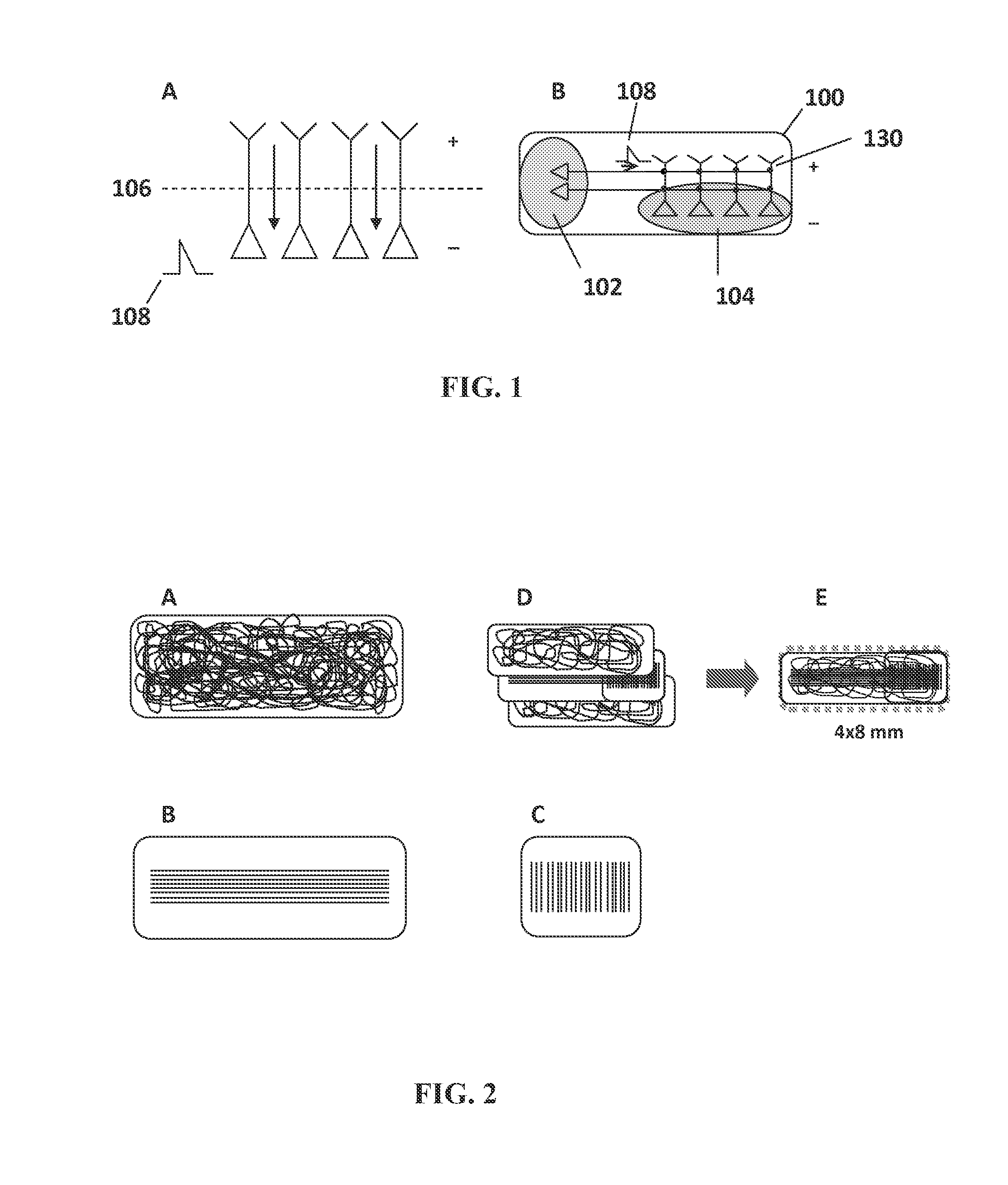
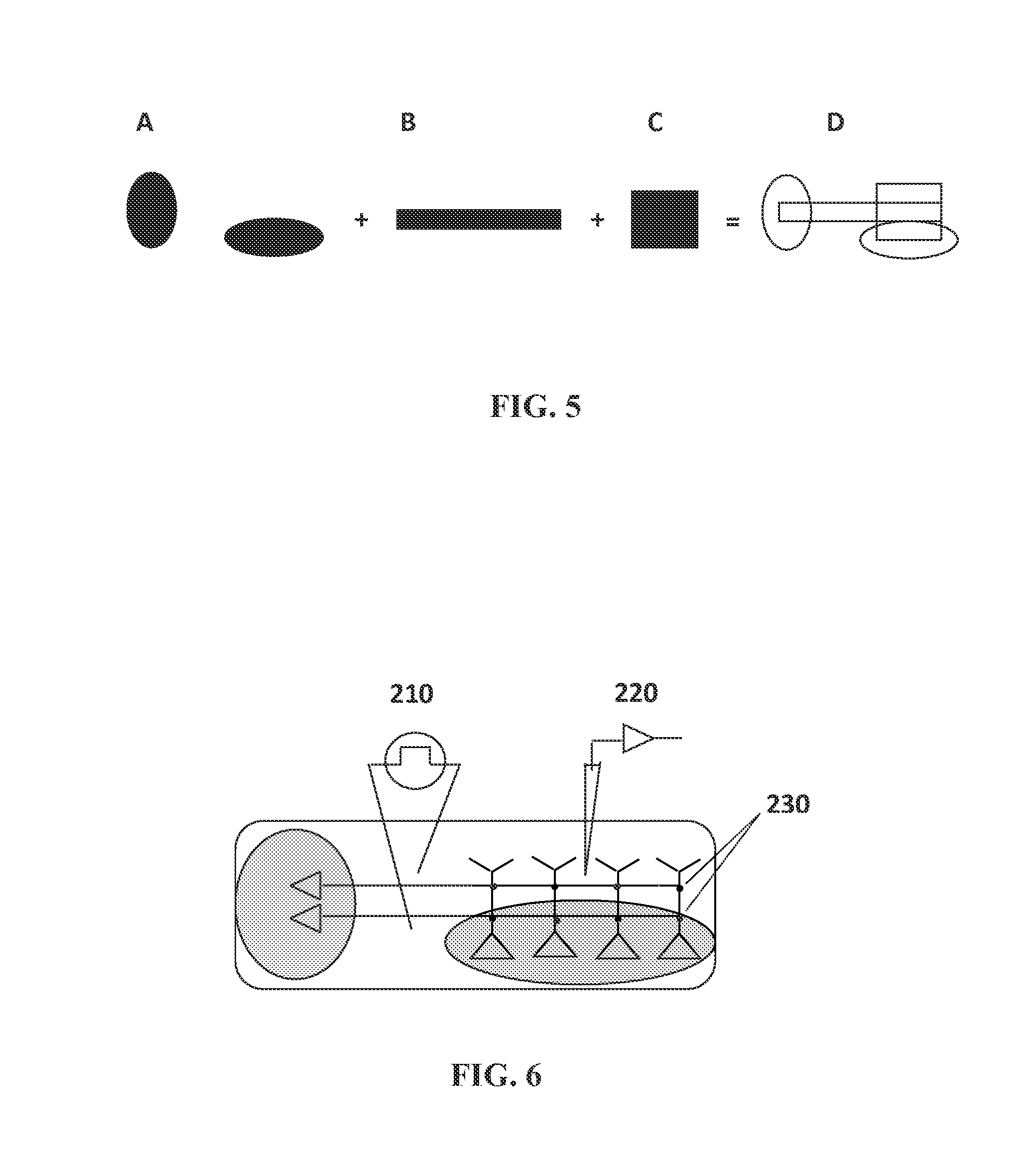
[0160]Nanofiber mats were treated to create a surface that included a passivating polymer (PEG) surrounding discrete domains for cell culture and growth. Silver halide photolithographic masks were used to create the discrete cell culture domains.
[0161]Work was performed using sterile procedures. Materials were sterilized with 70% ethanol and illuminated with UV light (306 nm) for 3 minutes per side. Prior to photopatterning, photoreactive nanofiber mats were incubated in a solution of polyethylene glycol (PEG, MW 10K, Sigma) for 2 hours, and dried in the dark.
[0162]Complimentary silver halide photolithographic films (CAD / Art Services, Inc., Bandon, Oreg.) were used to conjugate polyethylene glycol (PEG) outside of the desired cell growth and attachment areas (see FIG. 5 and FIG. 1B). The mask sets were provided in oval shapes. Complementary masks enabled simultaneous light exposure to both sides of the nanofiber mat and ensure mask alignment and opti...
example 3
Cytocompatibility of Scaffolding Material
[0168]To investigate the ability of prepared scaffolding material to support cell adhesion and proliferation, samples were sterilized with 70% ethanol for 24 hours, washed extensively with PBS (0.1M, pH 7.4) and exposed to UV light for 40 minutes (CL 1000 ultraviolet crosslinker, UVP). Scaffold materials were seeded with myoblasts (C2C12 cells, ATCC), endothelial cells (BAEC (Bovine aortic endothelial cells), Lonza Biosciences), and neural cells to observe the ability of the nanofibrous scaffolds to support growth of these cells.
[0169]Muscle myoblast cells. FIG. 3B shows attachment and growth of C2C12 cells on nanofibrous scaffolds. The scaffolds were prepared as described in Example 1. An amount of the formed nanofibrous mesh was immersed in 20 ml of 50 mg / ml PAA aqueous solution for 30 minutes in a quartz round dish (Quartz Scientific, Inc., Fairport Harbor, Ohio). Mild agitation was applied to remove the air bubbles trapped in the nanofibe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com