Mucoadhesive pharmaceutical composition and preparation method therefor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
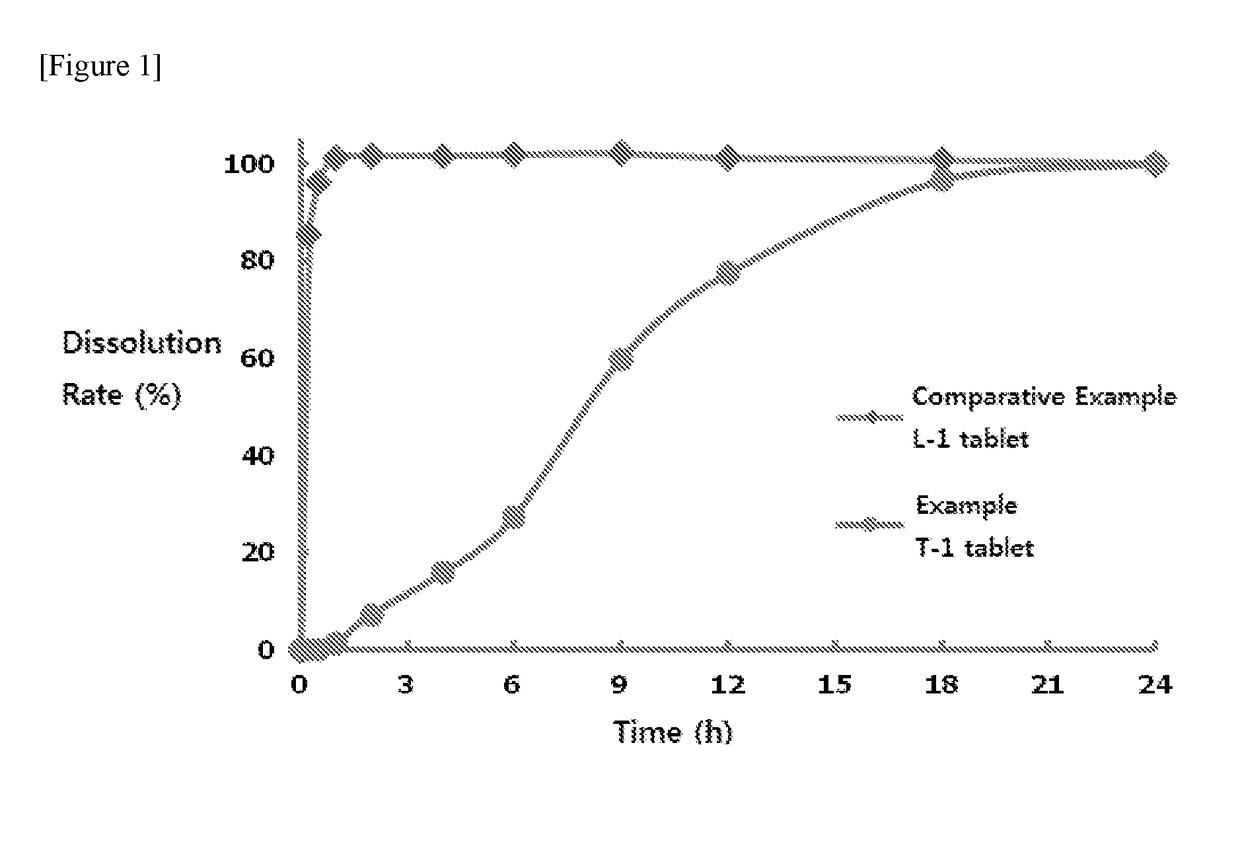
[0128]Dissolution test of an active ingredient from the formulation was conducted according to Method 2 Paddle Method of “36. Dissolution Test Method” in Korean Pharmacopoeia 8th edition (KP VIII). The eluent was selected from a buffer of pH 1.2, a buffer of pH 4.0, a buffer of pH 6.8, water and 0.1N HCl, and used in an amount of 900 ml. The test was conducted at 37° C. with 100 rotations per minute.
[0129]After dissolution, analysis of drug was conducted by using high performance liquid chromatography (HPLC). Analysis of drug was performed under conventionally known conditions.
[0130]For example, when the active ingredient was paliperidone, the following conditions were used for the analysis:
[0131]Mobile phase: A mixed solution of a solution prepared by dissolving 21.76 g of potassium phosphate monobasic in 4 L of water and adjusting the pH to 2.0 with phosphoric acid, and 1 L of acetonitrile
[0132]Column: XBridge phenyl. 150×4.6 mm, 3.5 μm
[0133]Flow rate: 1.0 ml / min
[0134]Column Tempe...
reference example 2
[0144]In order to test the mucoadhesiveness of the matrix-forming agent, the sample was prepared in disc form using a die to make disc for measuring IR (Infrared) spectrum. 100 mg of the sample was put into a die for preparing KBr disc and 5 to 10 tons of pressure was maintained for 3 minutes, and then a compressed disc with diameter of about 12 mm and thickness of about 0.75 mm was obtained.
reference example 3
veness Test
[0145]The in vitro test for mucoadhesiveness was conducted by utilizing a texture analyzer. A probe with diameter of 5 mm was used. The sample was fixed to the probe with double-sided tape, and cellulose acetate membrane filter was fixed at the lower part with double-sided tape and wetted with 100 μl of 3% mucin solution to simulate the environment of gastro-intestinal tract. Mucin was dissolved in a buffer of pH 1.2 or distilled water to make 3% solution for use. The measurement was made 1 minute after the wetting of the membrane. The descending speed of the probe was 0.5 mm / s, the pressing force of 1 N was maintained for 30 seconds, and then, the ascending speed was 0.5 mm / s, to measure mucoadhesiveness. The result is shown as adhesion force (N).[0146](Reference: Int J Pharm Sci Drug Res, 2011, 3, 84˜88, Eur J Pharm Biopharm. 2009. 71, 325-331)
[0147]The disc prepared according the method disclosed in Reference Example 2 was cut into a size of 2.5 mm×2.5 mm and attached ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com