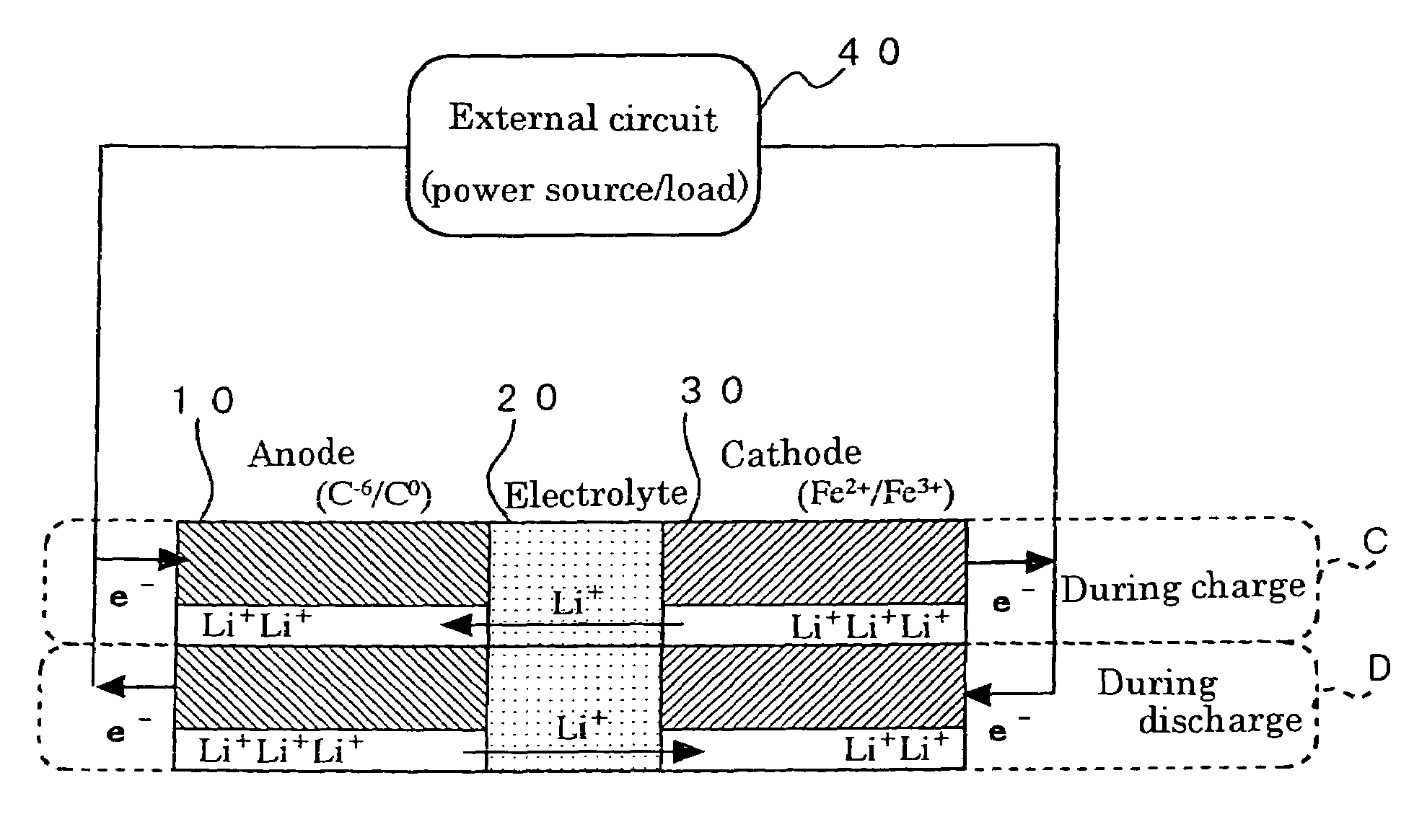
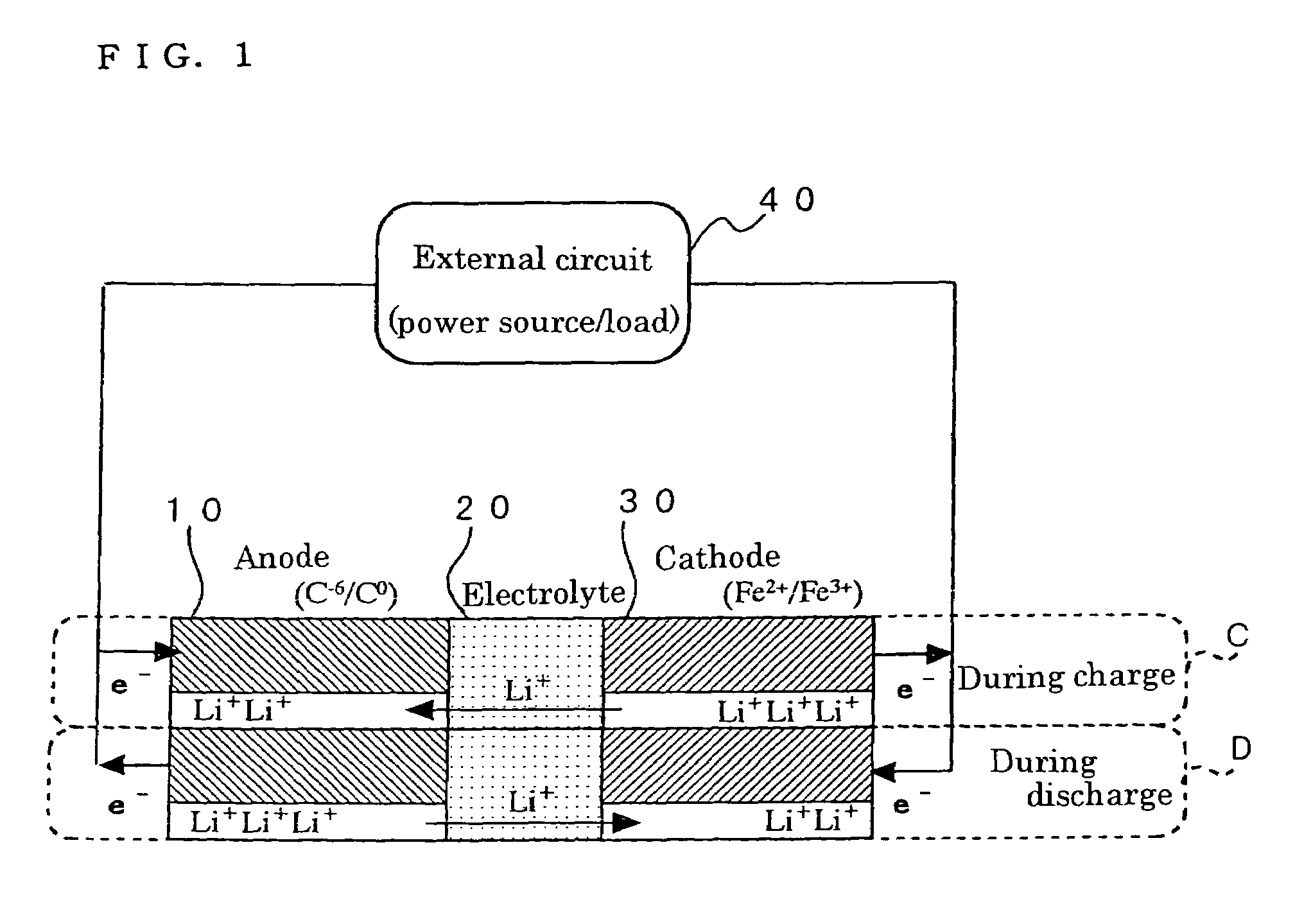
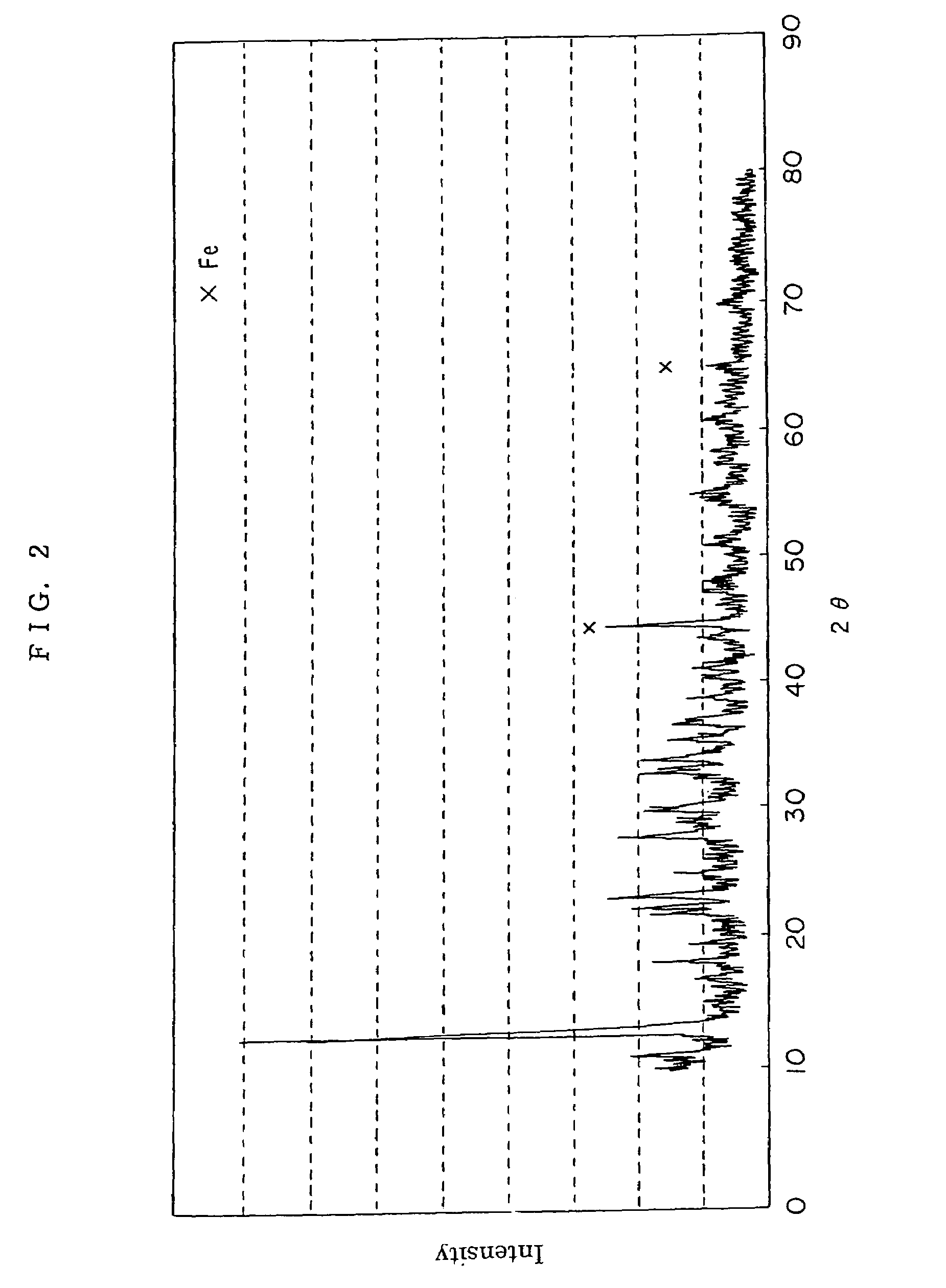
Method for preparing positive electrode material for secondary cell, and secondary cell
a technology of positive electrode material and secondary battery, which is applied in the direction of cell components, electrochemical generators, transportation and packaging, etc., can solve the problems of high production cost of cathode material, difficult control of cathode feed in a stoichiometric manner, and high cost of all secondary compounds. , to achieve the effect of high cathode performance, uniform and stable deposits of conductive carbon, and small diameter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0097](1) Preparation of Cathode Material:
[0098]A cathode material LiFePO4 was synthesized by the following procedure.
[0099]A mixture of 4.5 g of iron particles [under 325 mesh (44 μm) (purity 99.9%): product of The Nilaco Corporation] and 9.2901 g of 85% H3PO4 (product of Wako Pure Chemical Industries, Ltd.) was ground and reacted in an automatic grinding machine for two hours (when the viscosity of the mixture increased during grinding, distilled water was added to decrease the viscosity).
[0100]The reaction product was finely pulverized in a planetary ball mill for two hours. After addition of 3.381 g of LiOH.H2O, the mixture was further pulverized in the planetary ball mill for one hour. After removing water from the pulverized mixture with an evaporator, the mixture was vacuum-dried in a desiccator for two days. After addition of 0.6630 g of a coal pitch (product of Adchemco Corp., softening point: 250° C.), the mixture was manually ground for five minute to obtain a primary rea...
example 2
[0113](1) Preparation of Cathode Material:
[0114]A cathode material LiFePO4 was synthesized by the following procedure.
[0115]A mixture of 4.5 g of iron particles [under 325 mesh (44 μm) (purity 99.9%): product of The Nilaco Corporation] and 9.2901 g of 85% H3PO4 (product of Wako Pure Chemical Industries, Ltd.) was ground and reacted in an automatic grinding machine for two hours (when the viscosity of the mixture increased during grinding, distilled water was added to decrease the viscosity).
[0116]The reaction product (calcination precursor) was finely pulverized in a planetary ball mill for two hours. After addition of 3.381 g of LiOH.H2O, the mixture was further pulverized in a planetary ball mill for one hour. After removing water from the pulverized mixture with an evaporator, the mixture was vacuum-dried in a desiccator for two days.
[0117]The preliminary calcination was carried out in an atmosphere of nitrogen at 400° C. for five hours. After gas-cooled, the calcined product was...
example 3
[0128](1) Preparation of Cathode Material:
[0129]A cathode material LiFePO4 was synthesized by the following procedure.
[0130]A mixture of 1.5 g of iron powder [under 325 mesh (44 μm) (purity 99.9%): product of The Nilaco Corporation], 3.0967 g of 85% H3PO4 (product of Wako Pure Chemical Industries, Ltd.), and 1.1191 cc of hydrochloric acid (generally in the same number of moles as Li, Fe and PO4) was ground and reacted in an agate mortar for two hours. At this time, pure water was added as needed by dripping to decrease the viscosity of the mixture. After that, the mixture was placed in a beaker and ultrasonic wave was irradiated on the mixture for 40 minutes to accelerate the reaction. Then, the reaction mixture was pulverized in a planetary ball mill for two hours. After addition of 1.1270 g of lithium hydroxide, the mixture was further pulverized in the planetary ball mill for one hour. After removing water from the pulverized mixture with an evaporator, the mixture was dried in a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com