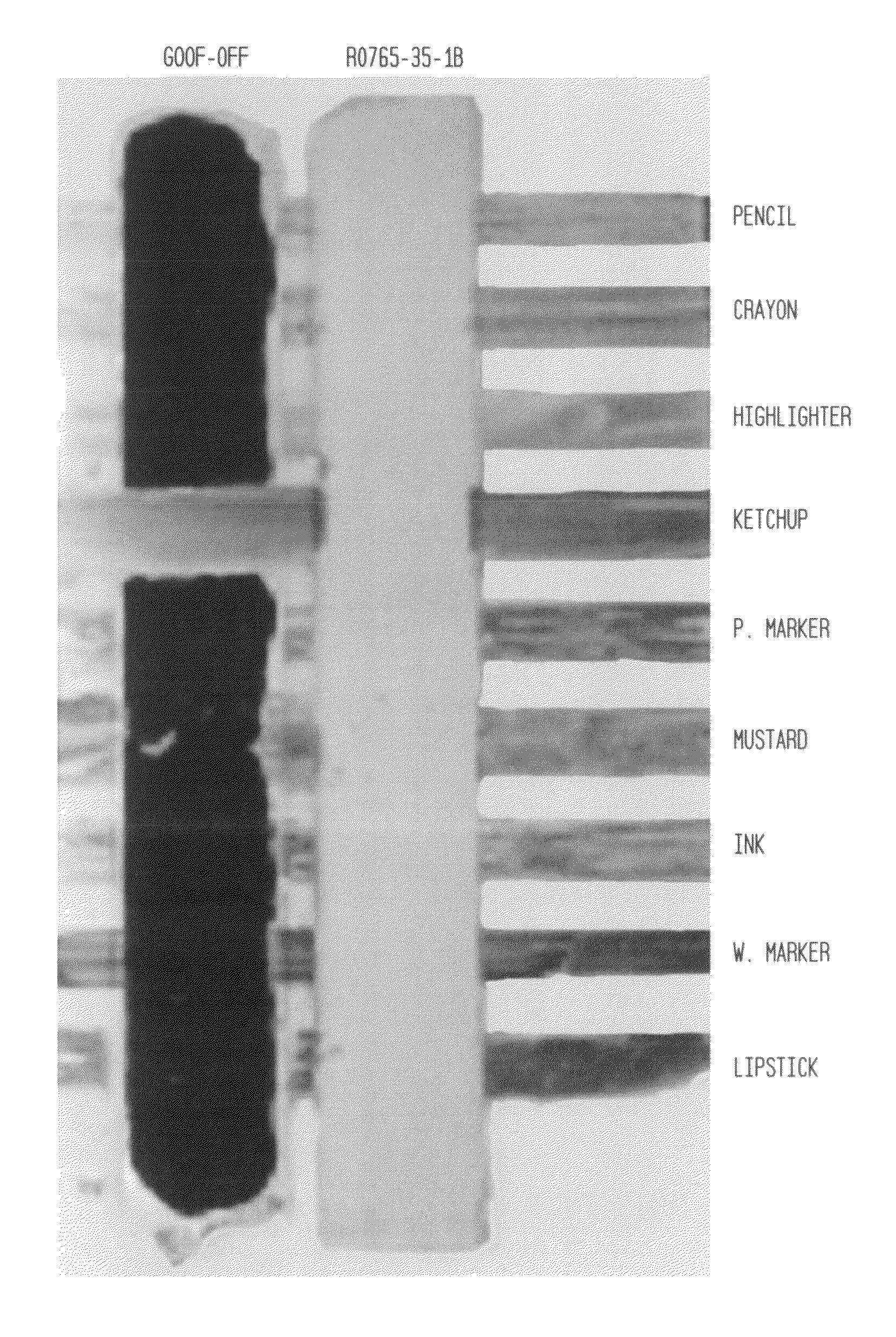
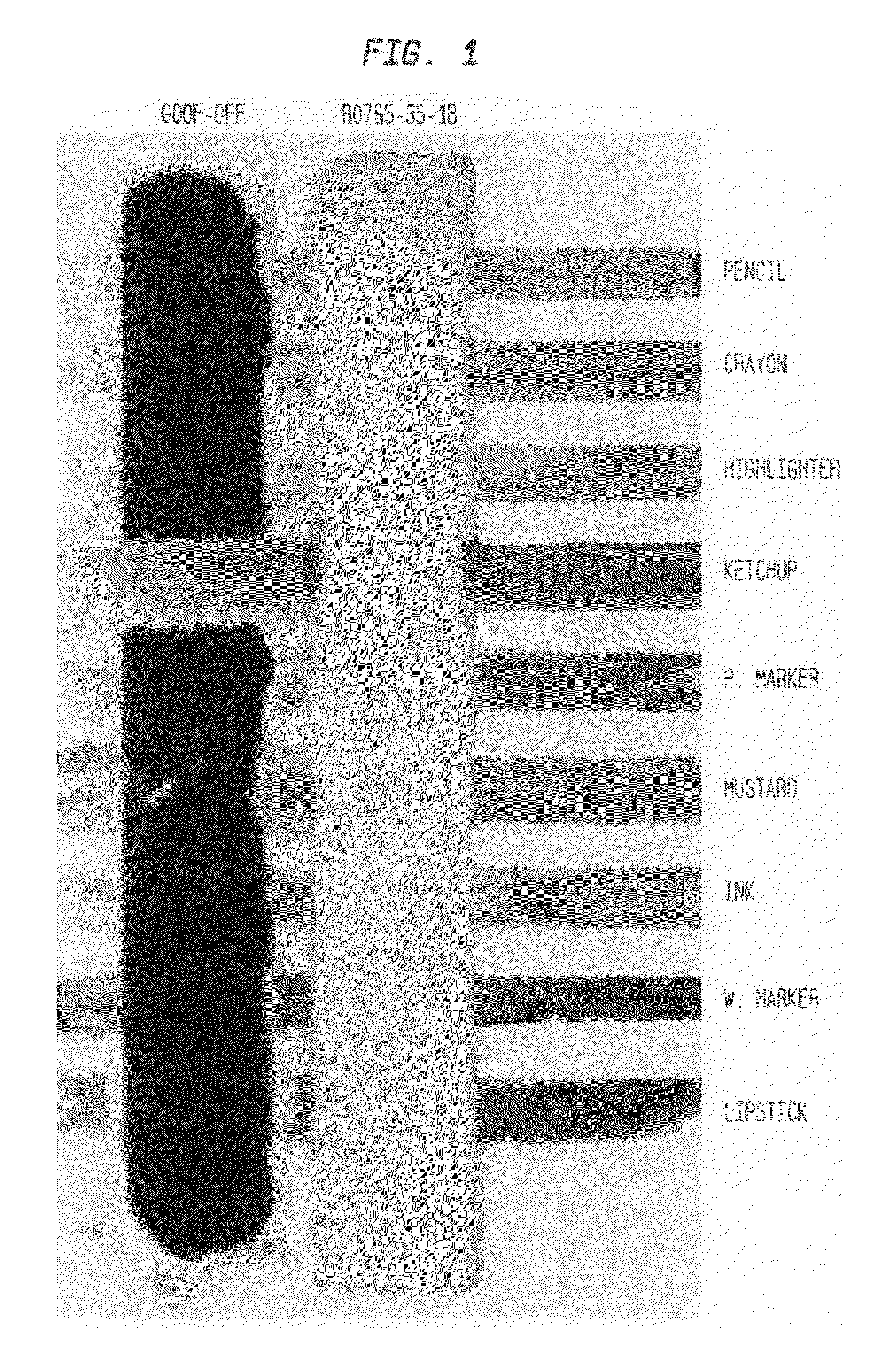
Cleaning compositions incorporating green solvents and methods for use
a technology of green solvents and cleaning compositions, applied in the field of cleaning compositions, can solve the problems of undesirable compositions, flashpoints that are also extremely flammable, and are not environmentally friendly, and achieve the effects of low vapor pressure, low odor, and high flash poin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Saturated Hydrocarbon (Isopar L™)
[0157]Isopar L™ is a high boiling mixture of saturated alkanes from Exxon with a described flash point of 68° C. and an evaporation rate of 0.06 (nBuAc=100). The solubility of UV Curable ink Nuvaflex™ 3003 (Cyan) from Zeller Gmellin was studied when the solvent was blended with a blend of MGN esters according to the present invention as shown in FIG. 1, below. Samples were prepared by dispensing aliquots (5 mL) of solvent blends into a series of vials with a progressive variation in MGN blend content. A drop of ink (0.012-0.015 g) was added to the solvent blend which sinks and deposits on the bottom of the vial. The solution was then mixed by repeated aspiration of 0.5 ml of the solvent with a micropipette 5 times. The solution was then allowed to stand for at least 24 hr. The ink is insoluble in neat Isopar L™. Increasing the MGN blend content increases the solubility of ink the blend. The cross over to complete solubility based on FIG. 10 occurs be...
example 2
Unstaurated Hydrocarbon (HiSol™ 70R)
[0158]Hisol™ 70R is an aromatic solvent with a described high evaporation rate compared to Isopar™ L. The solubility of UV Curable ink Nuvaflex™ 3003 (Cyan) from Zeller Gmellin was studied when the solvent (HiSol™ 70R) was blended with the MGN blend as photographically shown in FIG. 11. Samples were prepared by dispensing aliquots (5 mL) of solvent blends into a series of vials with a progressive variation in MGN blend content. A drop of ink (0.012-0.015 g) was added to the solvent blend which sinks and deposits on the bottom of the vial. The solution was then mixed by repeated aspiration of 0.5 ml of the solvent with a micropipette 5 times. The solution was then allowed to stand for at least 24 hr. It was observed that the ink is insoluble in HiSol™ 70R alone. Addition of the MGN blend of the present invention improves the solubility of ink with the crossover to complete solubility with an optically dense solution occurring between 30-40% MGN ble...
example 3
Oxygenated Solvents: Methyl Oleate (Fatty Acid Methyl Ester)
[0159]Fatty acid methyl esters sourced from various vegetable oils are touted to be more environmentally friendly which can replace some of the hazardous solvents used in the printing ink industry. Soy methyl esters (Methyl Soyate), Tall Oil mono-methyl esters are some examples of these environmentally-friendly solvents. These solvents, however, may not have the necessary solvency or the dissolution rates that are necessary at times for application in the printing industry. Methyl Oleate (C18 insaturated FAME) is one of the key ingredients of most such mono-methyl esters derived from disparate sources. Methyl oleate from Novance Inc (Phytorob™ 960.65) was used in this study. The solubility of UV Curable ink Nuvaflex™ 3003 (Cyan) from Zeller Gmellin was studied when the solvent (Me-Oleate) was blended with a particular dibasic ester blend AGS (dimethyl glutarate / succinate / adipate blend=63 / 24 / 13) as photographically shown in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com