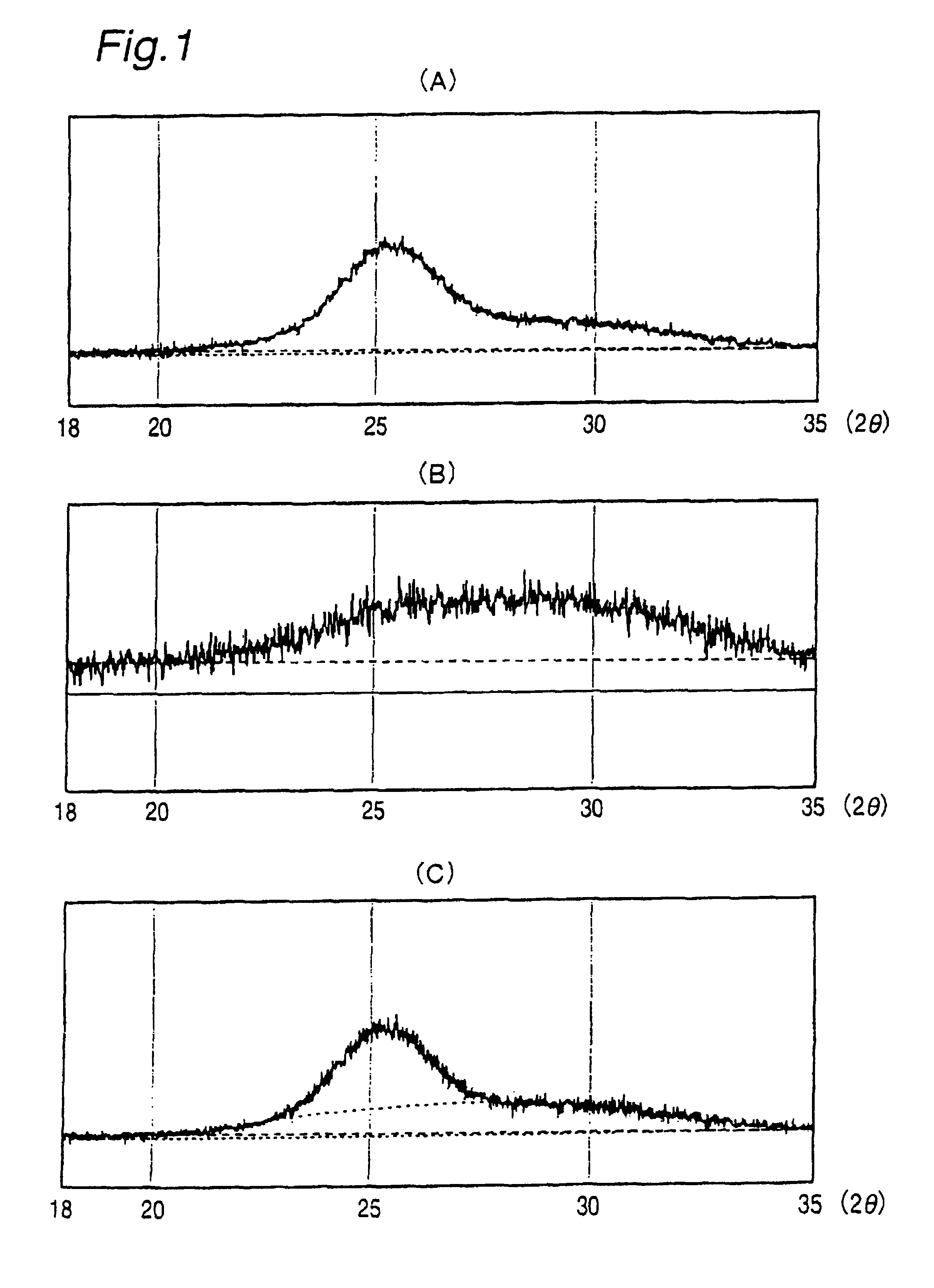
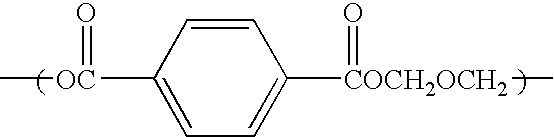
Catalyst for polyester production, process for producing polyester using the catalyst, polyester obtained by the process, and uses of the polyester
a technology of catalyst and polyester, which is applied in the direction of physical/chemical process catalysts, physical/chemical processes, electrography/magnetography, etc., can solve the problems of high production cost of polyester, inferior polyester produced by the use of antimony compound as a polycondensation catalyst, and high cost of germanium compound, etc., to achieve high catalytic activity, low acetaldehyde content, and high quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0725]The present invention is further described with reference to the following examples, but it should be construed that the invention is in no way limited to those examples.
example 495-1
Preparation of Solid Titanium Compound
[0726]Deionized water of 500 ml was weighed out and introduced into a 1000 ml glass beaker. The deionized water in the beaker was cooled in an ice bath, and thereto was dropwise added 5 g of titanium tetrachloride with stirring. When production of hydrogen chloride stopped, the beaker containing the reaction solution was taken out of the ice bath, and 25% aqueous ammonia was dropwise added with stirring, to adjust pH of the solution to 8. The precipitate of titanium hydroxide produced was separated from the supernatant liquid by centrifugation of 2500 revolutions for 15 minutes. Then, the resulting titanium hydroxide precipitate was washed five times with deionized water. After the washing, solid-liquid separation was carried out by centrifugation of 2500 revolutions for 15 minutes. The washed titanium hydroxide was vacuum dried at 70° C. under a pressure of 10 Torr for 18 hours to remove water content, whereby a solid titanium compound was obta...
example 495-2
Preparation of Titanium-containing Solid Compound
[0730]Deionized water of 500 ml was weighed out and introduced into a 1000 ml glass beaker. To the deionized water, 0.15 g of anhydrous magnesium hydroxide was added to give a dispersion. The dispersion in the beaker was cooled in an ice bath, and thereto was dropwise added 5 g of titanium tetrachloride with stirring. The liquid became acidic, and the magnesium hydroxide was dissolved. When production of hydrogen chloride stopped, the beaker containing the reaction solution was taken out of the ice bath, and 25% aqueous ammonia was dropwise added with stirring, to adjust pH of the solution to 8. The precipitate of titanium-containing complex hydroxide produced was separated from the supernatant liquid by centrifugation of 2500 revolutions for 15 minutes. Then, the precipitate of titanium-containing complex hydroxide was washed five times with deionized water. After the washing, solid-liquid separation was carried out by centrifugation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| diffraction angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com