DL-amygdalic acid preparing method
A technology of mandelic acid and mandelonitrile, which is applied in the field of preparation of DL-mandelic acid, can solve the problems of difficult treatment, large amount of three wastes and high cost, and achieves the effects of high reaction conversion rate, reduction of pollutants and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
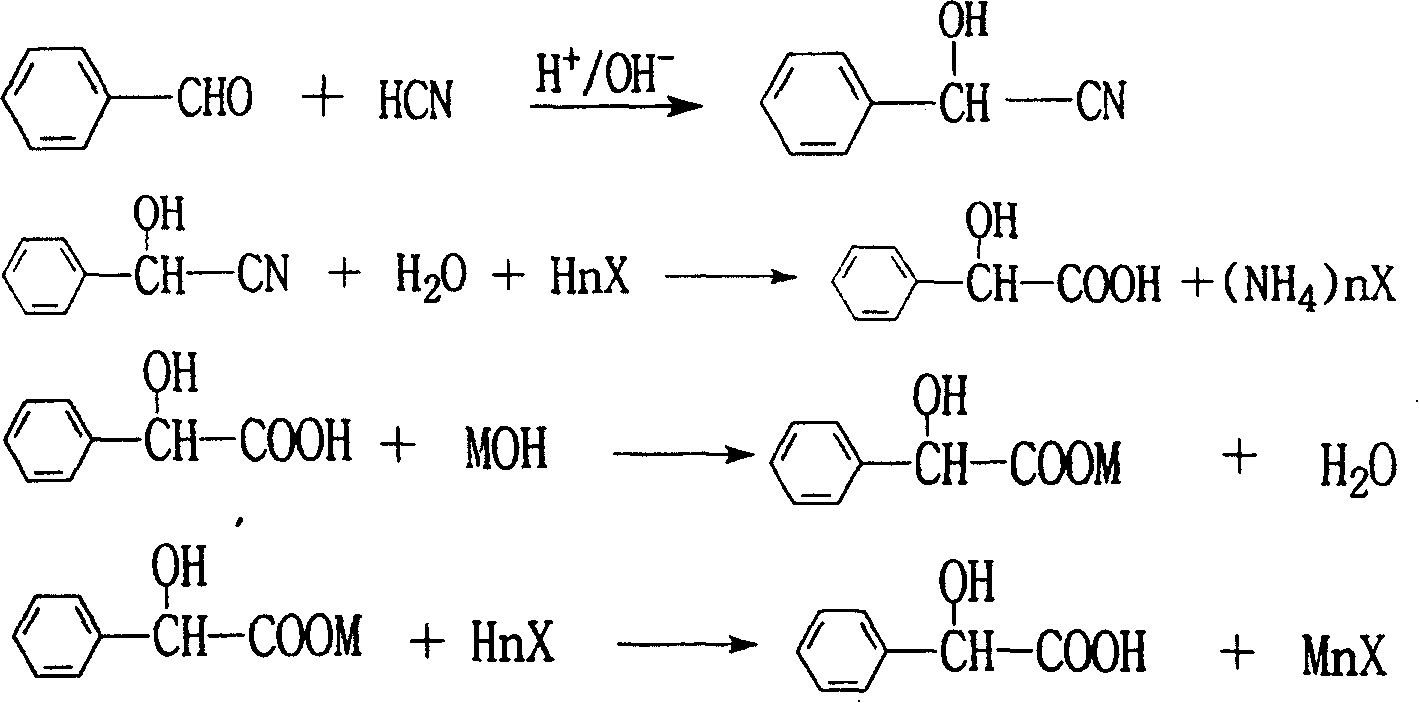
[0028] In the 1000L reactor, add benzaldehyde (the weight percentage content is 99%) 450kg, water 450kg, the concentrated sulfuric acid catalyst 7.5kg, pass into the hydrogen cyanide gas that the weight percentage content is 8% at 70~80 ℃, flow control and Calculate the weight of hydrogen cyanide introduced. When feeding hydrogen cyanide weight to reach 120kg, stop feeding. The incubation reaction was completed in 0.5 hours. Transfer the cyanidation reaction solution to a 2000L hydrolysis reactor, slowly add 255kg of concentrated sulfuric acid at 80-90°C, and complete the addition and keep warm for 2 hours to complete the reaction. Analysis of the DL-mandelic acid content in the reaction solution is 47.2% (HPLC), add water 600L, be cooled to 10 ℃ of crystallization, centrifuge, get DL-mandelic acid crude product 630kg, water content 7%, count DL-mandelic acid content in dry basis It is 96.2% (acid-base titration), and the yield in one reaction is 88.2%. Mother liquor 1150L,...
Embodiment 2
[0031] In the 1000L reactor, add benzaldehyde (the weight percentage content is 99%) 450kg, water 450kg, the concentrated sulfuric acid catalyst 7.5kg, pass into the hydrogen cyanide gas that the weight percentage content is 8% at 70~80 ℃, flow control and Calculate the amount of hydrogen cyanide introduced. When feeding hydrogen cyanide weight to reach 120kg, stop feeding. The incubation reaction was completed in 0.5 hours. Transfer the cyanidation reaction solution to a 2000L hydrolysis reactor, slowly add 255kg of concentrated sulfuric acid at 80-90°C, and complete the addition and keep warm for 2 hours to complete the reaction. The content of DL-mandelic acid in the analyzed reaction solution was 47.2% (HPLC).
[0032] In the hydrolysis mother liquor of embodiment 1, add liquid ammonia 27.0kg, mother liquor crystallizes at 10 ℃ after concentrating under reduced pressure, centrifugation separates by-product ammonium sulfate, by-product weighs 320kg after drying, content 9...
Embodiment 3
[0035] Carry out feeding operation as in Example 2, the difference is that the liquid ammonia is changed into 140kg of 42% sodium hydroxide solution by weight percentage, after the mother liquor is concentrated, it is filtered while hot to remove sodium sulfate, and then the filtrate is cooled to 10°C for crystallization , by-product ammonium sulfate is centrifugally separated, after drying, the by-product ammonium sulfate weighs 296kg, and the content is 90%, and the remaining filtrate replaces the added clear water in the hydrolysis process for recycling, and the crystallization of the hydrolyzate obtains DL-mandelic acid crude product 660kg, with a water content of 8%, The DL-mandelic acid content is 93% on a dry basis. The average yield of DL-mandelic acid reaction was 88.4%. The mother liquor is recycled according to the above steps.
[0036] Add above-mentioned DL-mandelic acid crude product in the recrystallization mother liquor that example 2 obtains, add gac 12kg, de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com