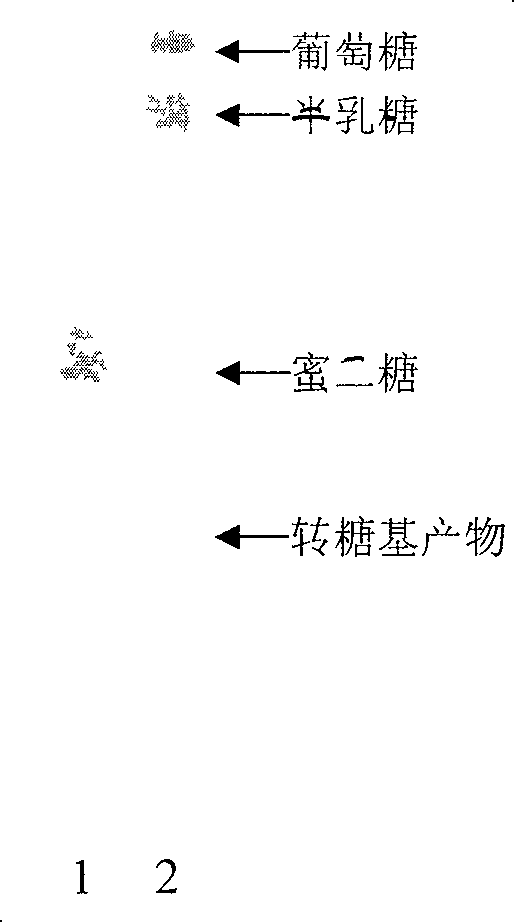
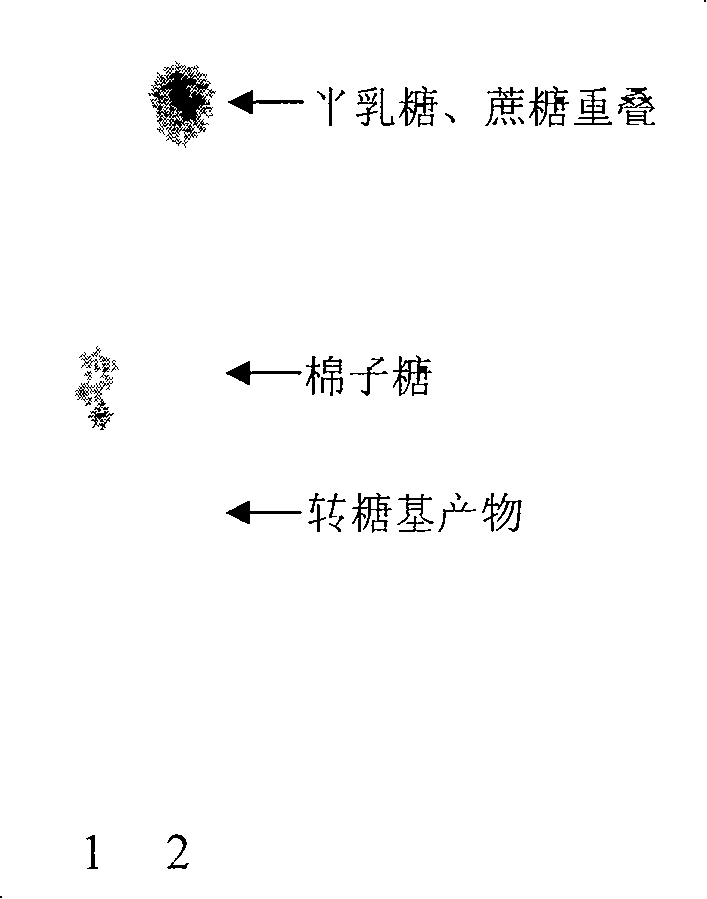
Transglycosyl alpha-galactoglucosidezyme gene
A technology of galactosidase and transglycosidation, which is applied in genetic engineering, plant genetic improvement, enzymes, etc., can solve the problem of lack of bifidobacterium breve transglycosyl α-galactosidase gene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Extraction of Bifidobacterium breve (Bifidobacterium breve) ATCC 15700 Chromosomal DNA
[0025] Inoculate Bifidobacterium breve (Bifidobacterium breve) ATCC 15700 in 5mL MRS medium, culture anaerobically at 37°C for 24 hours, take 1.5ml of the culture, centrifuge at 12,000 rpm for 2 minutes, and suspend the obtained pellet in 565μL of TE buffer Add lysozyme to a final concentration of 10 mg / ml, incubate at 37°C for 30 minutes; add 30 μL of 10% (mass volume ratio) sodium dodecyl sulfate (SDS) and 5 μL of 20 mg / mL proteinase K, mix well, and Incubate at 37°C for 1 hour; add an equal volume of phenol / chloroform / isoamyl alcohol (25:24:1, v / v / v), mix well; centrifuge at 12,000 rpm for 5 minutes, and transfer the supernatant to a fresh Add an equal volume of phenol / chloroform / isoamyl alcohol to the tube, and mix well; centrifuge at 12,000 rpm for 5 minutes, transfer the supernatant to a new tube, add 2 times the volume of absolute ethanol, and mix gently until the D...
Embodiment 2
[0028] Example 2 Cloning of highly conserved DNA fragment of B breve ATCC 15700 α-galactosidase gene
[0029] The DNA sequences of the α-galactosidase genes of different strains were retrieved from GenBank, the sequence homology was compared with the online software Clustal W, and a pair of degenerate PCR primers were designed using the primer design software Primer5.0:
[0030] Wtz-F: 5'-(TC)TI AA(TC)A(AC)(N)TGG GA(AG)G-3'
[0031] Wtz-R: 5'-(GA)TC CCA(CT)TT(N)A(CT)(GA)TA(GA)TC-3'
[0032] Using the extracted B breve ATCC 15700 chromosomal DNA as a template, PCR was carried out with the above primers. The total volume of the PCR amplification system is 50 μL, containing 35 μL of ultrapure water, 5 μL of 10×PCR buffer, dNTP 2.4 mmol / L, primers 1 μmol / L, MgCl 2 1.5mmol / L, template DNA 100ng, TaKaRa Taq 2 5U. PCR amplification conditions: pre-denaturation at 95°C for 5 minutes; reaction for 28 cycles (denaturation at 95°C for 1 minute, annealing at 52°C for 1 minute, extensi...
Embodiment 3
[0043] Example 3 Cloning of B.breve ATCC 15700α-galactosidase whole gene
[0044] (1) Construction of B breve ATCC 15700 partial gene library
[0045] After the B breve ATCC 15700 chromosomal DNA is extracted, six sets of complete enzyme digestion are carried out at the same time. The six sets of enzymes are BamH I-HindIII, BamH I-Nde I, BamH I-Xba I, HindIII-Nde I, HindIII-Xba I, BamH I (TaKaRa). After electrophoresis, the membrane was transferred, and the cloned 457bp DNA fragment was used as a probe for Southern hybridization. The results showed that after the B breve ATCC 15700 chromosomal DNA was digested with BamH I and Nde I, the fragment that could hybridize with the probe was about 6kb, which could be used to construct a partial gene library. It was estimated by experiments that about 81.3% of the recombinant bacteria in some gene libraries had foreign fragments, and the library was successfully constructed.
[0046] (2) Screening, verification and sequence determi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com