Basic culture medium and culture method for circulating tumor cells
A technology of basal medium and tumor cells, applied in the fields of medicine and biology, basal medium and culture of circulating tumor cells, can solve the problems of difficult cell enrichment and improper culture conditions, and achieve the effect of improving the accuracy of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of lyophilized powder of Streptomyces roseus fermentation broth
[0050] 1. The source of bacteria
[0051] Purchased from China Industrial Microorganism Culture Collection and Management Center: Streptomyces roseofuscus (Strain collection number: ACCC40123 (Platform number: bio-52663), hereinafter referred to as laboratory abbreviation SR), it is known that it can be used to inhibit Gram Gram-positive bacteria, Gram-negative bacteria and filamentous fungi.
[0052] 2. Preparation of culture medium
[0053] 1) Seed medium (g / L):
[0054] Glucose 20.0g, peptone 6.0g, NaCl 10.0g, pH 7.0.
[0055] 2) ISP2 medium (medium proposed by the International Streptomyces Planning Conference):
[0056] Yeast extract 4.0g, malt extract 10.0g, glucose 4.0g, after dissolving, adjust the pH and dilute to 1.0L, sterilize by high pressure (1.034×105Pa) at 115°C for 30 minutes (glucose will be decomposed into For the cultivation of harmful microorganisms of sugar...
Embodiment 2
[0059] Example 2 Cytotoxicity test of lyophilized powder of Streptomyces roseus fermentation broth
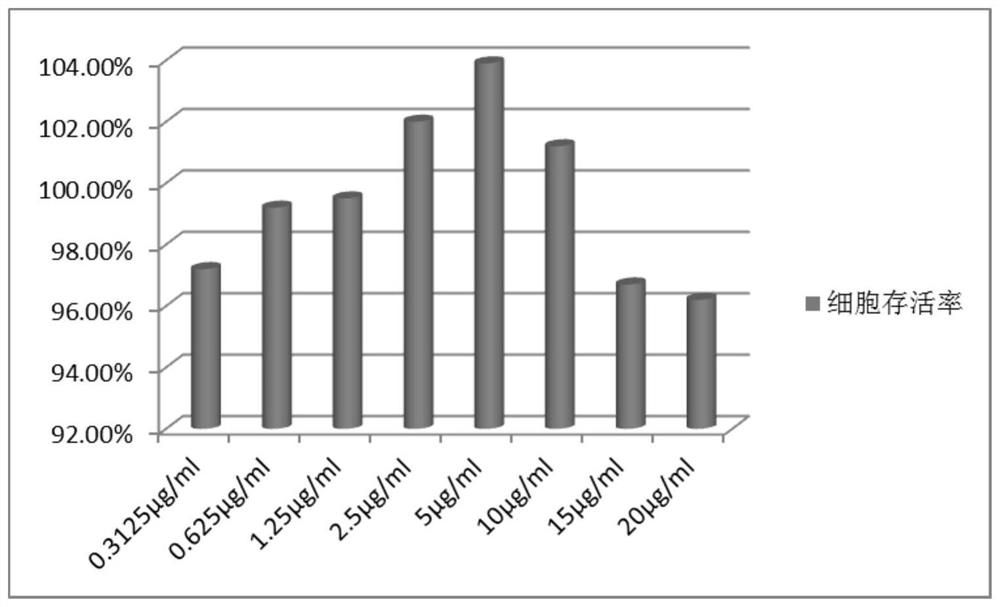
[0060] After HEK293 cells (human embryonic kidney cells) were inoculated in culture flasks, RPMI1640 medium (Gibco) containing 10% fetal bovine serum was used at 37°C, 5% CO. 2 Cultivated in the environment for 12-72h. Take the cells in the logarithmic growth phase, resuspend the cell suspension in medium, and adjust the density to 1×10 5 1 / mL, add 100 μL PBS to the peripheral wells of the 96-well culture plate using a multi-channel pipette, and add 100 μL to the remaining wells at a density of 1×10 5 cells / mL of cell suspension. Cells at 37°C, 5% CO 2 Incubate in the incubator for 24h, aspirate the medium, add 100μL of 0μg / ml (control group), 0.3125μg / ml, 0.625μg / ml, 1.25μg / ml, 2.5μg / ml, 5μg / ml, 10μg / ml to each well ml, 15μg / ml, 20μg / ml concentration of SR fermentation broth lyophilized powder medium. After being placed in the incubator for 24 hours, the medium was remove...
Embodiment 3
[0068] Example 3 Composition and use steps of the kit
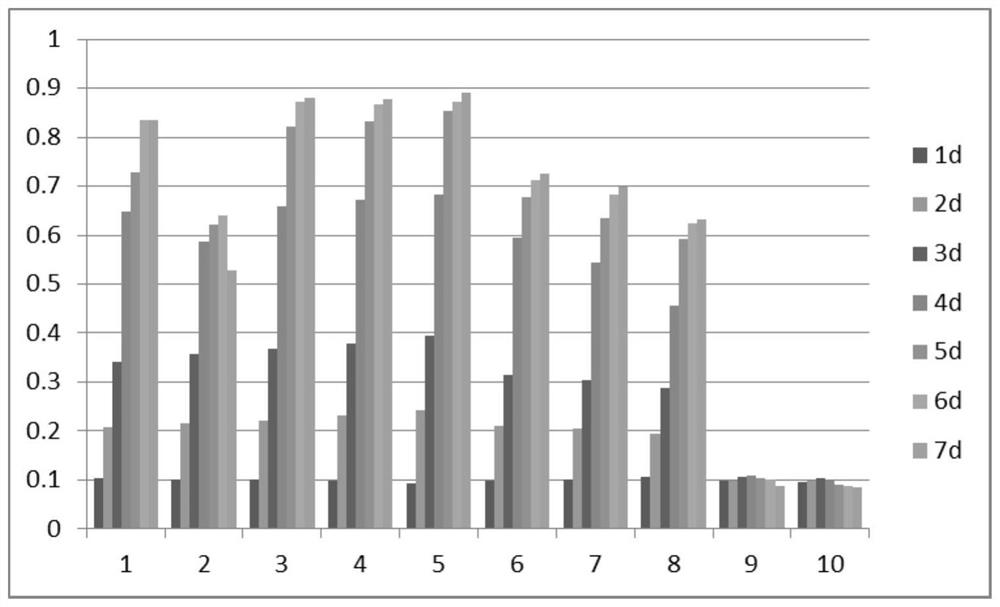
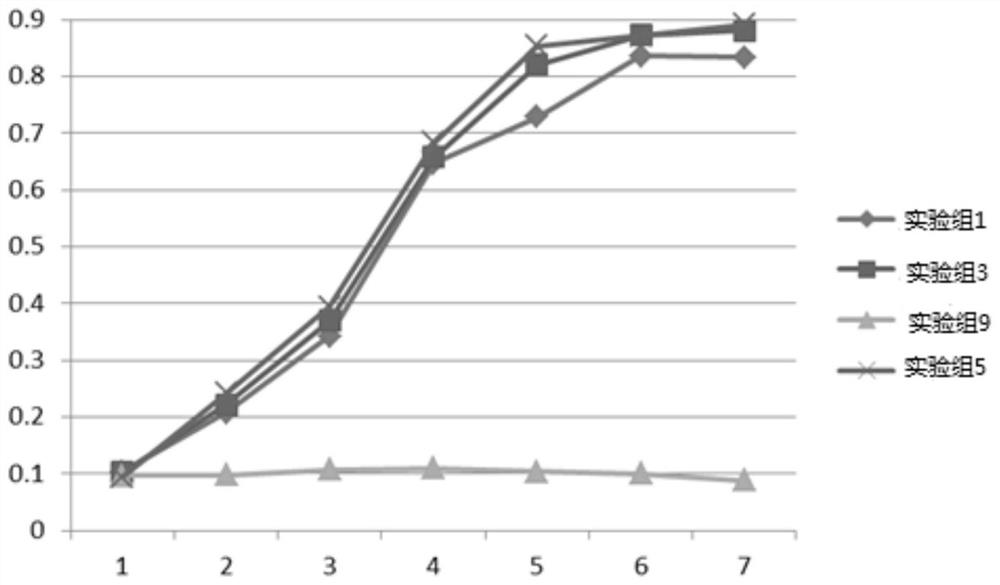
[0069] The kit of the invention mainly includes a circulating tumor cell basal medium, which specifically includes the following components: RPMI1640 / F12 medium, 8ng / mL basic fiber growth factor FGF2, 6μg / mL human recombinant insulin, 1mM pyruvate Sodium (antioxidant), 11mg / L transferrin, 0.7mM N-acetylcysteine, 9mM nicotinamide (vitamin B3 derivative), 0.6mg / mL taurine, 50U / mL penicillin, 50μg / mL streptomycin, 5μg / ml Streptomyces roseus (SR) fermentation broth (lyophilized powder); wherein RPMI1640 / F12 medium is F12 medium (Gibco, Item No.: 11765062) and RPMI1640 medium (Gibco, Item No.: A1049101 ) were prepared 1:1.
[0070] The basal medium can also be used to prepare a complete medium. The complete medium can be composed of basal medium and serum, wherein the serum can be obtained after coagulation and centrifugation of whole blood, or commercial fetal bovine serum.
[0071] The present invention can be applied to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com