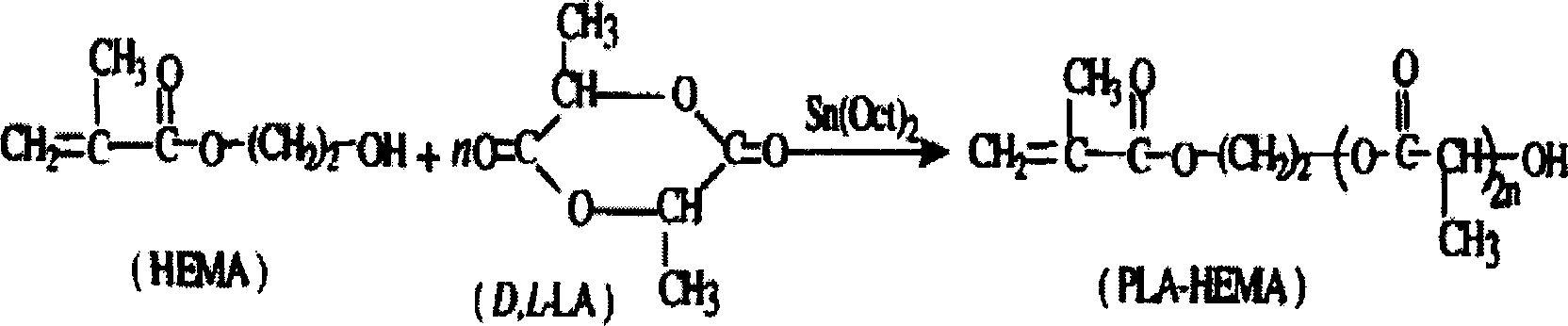
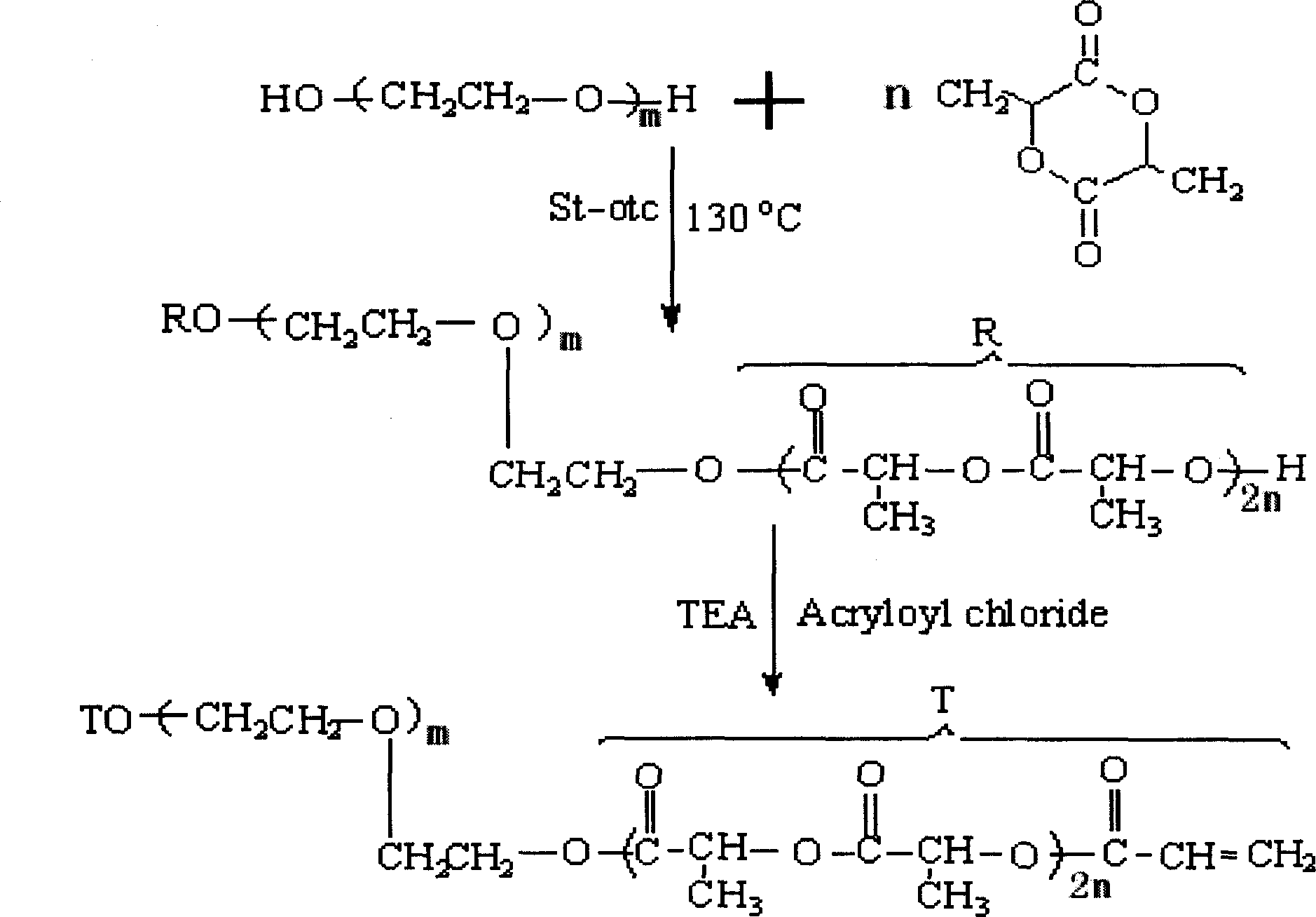
Biodegradable active medical tissue adhesive and its preparation method
A tissue adhesive and biodegradable technology, used in pharmaceutical formulations, surgical adhesives, applications, etc., can solve the problems of releasing toxic monomers, unfavorable to cells, and reducing the adsorption capacity of biocompatible calcium ions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] 0.22g macromonomer (MC-5), 0.34g degradable cross-linking agent were dissolved in 0.44g vinylpyrrolidone monomer, and 0.0075g benzoyl peroxide and 30ppm hydroquinone were added to obtain uniform and stable Clear Solution Component A.
[0083] When bonding skin lacerations, the operator first cleans up the wound thoroughly, without anesthesia and hemostasis, and uses the forefinger and thumb of the left hand to press lightly at a distance of 0.16 to 0.18 cm from the wound edge, which plays the role of oppression and hemostasis, and can Align the edges together. Drop 0.0050g of N,N-dimethyl-p-aniline into component A, mix well within 1min, and drop it on the wound so that it can completely cover the wound. After 2min to 3min, two fingers can slowly Loosen in a criss-cross pattern, and cover the wound with gauze after observing that the wound is no longer bleeding or splitting. After testing: the wound was completely absorbed and degraded after 8 weeks, and tissue sectio...
Embodiment 2
[0085] Dissolve 0.20g macromer (MC-3), 0.40g degradable cross-linking agent in 0.40g vinylpyrrolidone monomer, add 0.0075g benzoyl peroxide and 30ppm hydroquinone and mix to obtain uniform and stable Clear Solution Component A. In ophthalmic surgery, after the small and irregular corneal wound is sutured, add 0.005g N, N-dimethyl-p-aniline to component A, mix well within 1mim, drop into the wound that cannot be completely closed after suturing, 2min-3min Form a transparent film, which can effectively prevent the leakage of aqueous humor.
Embodiment 3
[0087] Dissolve 0.20g macromer (MC-5), 0.35g degradable cross-linking agent in 0.45g vinylpyrrolidone monomer, add 0.0075g benzoyl peroxide and 30ppm hydroquinone to obtain uniform and stable Clear solution.
[0088]New Zealand white rabbits were anesthetized and fixed in the supine position. According to the principle of aseptic technique, a midline incision was made on the upper abdomen to enter the abdominal cavity, and the upper part of the small intestine was cut off. Add 0.0050 g of N,N-dimethyl-p-aniline to the component one solution, and mix well within 1 min. Use it to glue the intestinal tube and the edge of the membrane. After 3 minutes, check that there is no leakage at the anastomosis, return the intestinal tube to the abdominal cavity, and close the abdomen. Absorption and degradation were basically complete after 12 weeks. Tissue sections showed that the material had good biocompatibility.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com