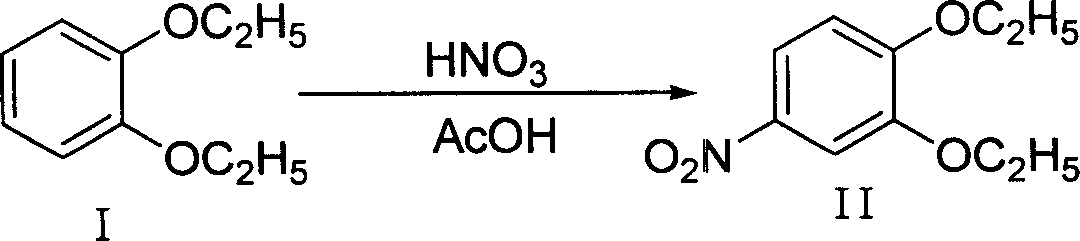
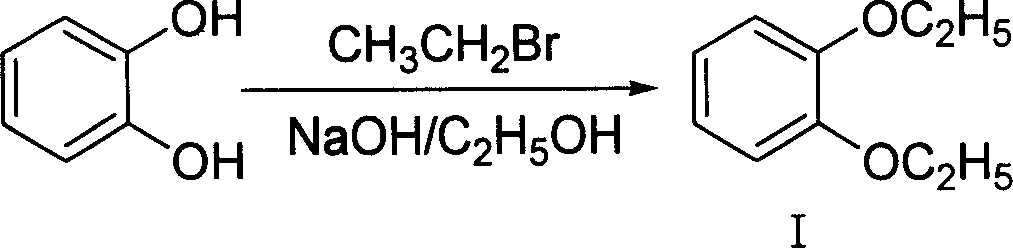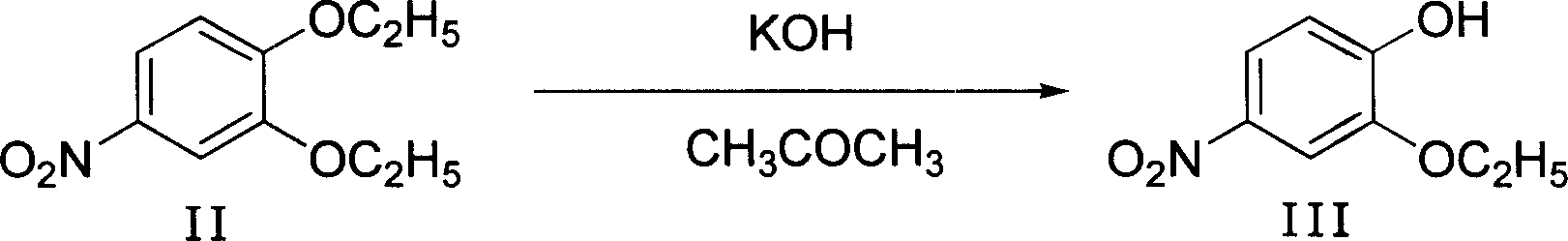Method of preparing 4-hydroxy-6-decyloxy-7-ethoxy-3-quinoline carboxylic acid ethyl ester
A technology of ethyl quinoline carboxylate and ethoxy, which is applied in a new field of preparation, can solve the problems of harsh reaction conditions and equipment, large waste of intermediate reactions, and large environmental pollution, and meet the requirements of avoiding reaction conditions and equipment Harsh, mild reaction conditions, wide-ranging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A, the preparation of pyrocatechol diethyl ether (I)
[0027]
[0028] Add catechol (11g, 0.1mol) in the 250ml three-neck flask that reflux condenser, thermometer and magnetic stirring device are housed, 30% sodium hydroxide aqueous solution (20ml, about 0.2mol), ethanol (30ml) and Sodium iodide (0.1g), heat the solution, then slowly drop into the mixture of bromoethane (43.6g, 0.4mol) and ethanol (30ml), keep the oil temperature at 60°C, reflux for 15-18 hours, TLC (thin layer chromatography) showed that the reaction of catechol was complete, let it stand for cooling, separated the upper oil layer, evaporated unreacted bromoethane and solvent ethanol under reduced pressure, and crystallized the remaining oily matter at about 0°C to obtain the crude product . The crude product was recrystallized in ethanol to obtain I (15.0 g), yield 90.3%. The melting point of the product is 42-44°C. In this example, the reason why ethanol is used as the solvent is that the high ...
Embodiment 2
[0049] Other steps are identical with embodiment 1, just the preparation method of the pyrocatechol diethyl ether (I) of A step is as follows:
[0050] Add pyrocatechol (11g, 0.1mol) in the 250ml three-neck flask that reflux condenser, thermometer and magnetic stirring device are housed, 30% sodium hydroxide aqueous solution (50ml, about 0.5mol), ethanol (30ml) and Sodium iodide (0.1g), stirred at room temperature, then slowly added diethyl sulfate (61.6g, 0.4mol) dropwise, kept the oil temperature at 100°C, refluxed for 5-8 hours, TLC (thin layer chromatography) showed that o-phenyl After the reaction of the diphenol is complete, let it stand for cooling, separate the upper oil layer, remove the solvent ethanol under reduced pressure, and crystallize the remaining oil at about 0°C to obtain the crude product. The crude product was recrystallized in ethanol to obtain I (15.7 g), yield 94.5%. The melting point of the product is 42-44°C.
Embodiment 3
[0052] Other steps are identical with embodiment 1, just the preparation method of the pyrocatechol diethyl ether (I) of A step is as follows:
[0053] Add pyrocatechol (11g, 0.1mol) in the 250ml three-neck flask that reflux condenser, thermometer and magnetic stirring device are housed, 15% sodium hydroxide aqueous solution (100ml, about 0.5mol), ethanol (30ml) and Sodium iodide (0.1g), stirred at room temperature, then slowly added diethyl sulfate (61.6g, 0.4mol) dropwise, kept the oil temperature at 100°C, refluxed for 5-8 hours, TLC (thin layer chromatography) showed that o-phenyl After the reaction of the diphenol is complete, let it stand for cooling, separate the upper oil layer, remove the solvent ethanol under reduced pressure, and crystallize the remaining oil at about 0°C to obtain the crude product. The crude product was recrystallized in ethanol to give I (15.3 g), yield 92.1%. The melting point of the product is 42-44°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com