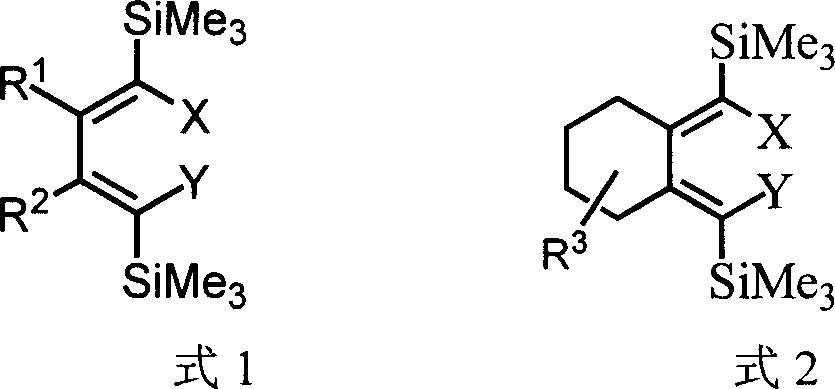
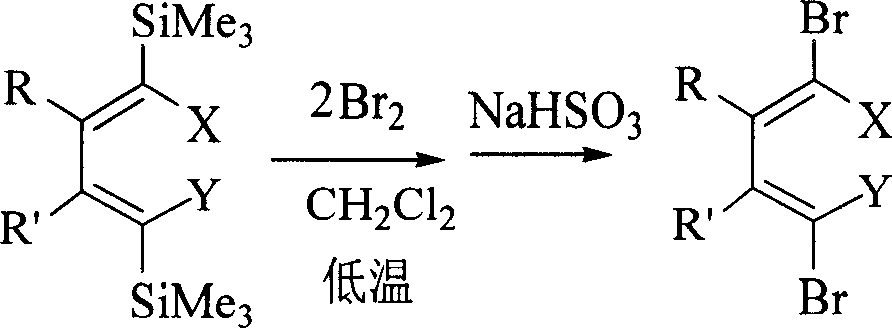
Method for preparing 1,1,4,4-tetrahalogenated-1,3-butadiene derivative by 1,4-dihalogenated-1,4-di(trisilicon methyl radical)-1,3-butadiene derivatives
A technology of trimethylsilyl and butadiene, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry, etc. Simple and easy operation, convenient for industrialization, and the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] One of the I class compounds in the structural formula (R 1 =R 2 =n-Bu): Synthesis of (5Z,6Z)-5,6-bis(dibromomethylene)decane:
[0027] In a 25 ml round bottom flask, dissolve 1 mmol of (5Z,6Z)-5,6-bis[(trimethylsilyl)bromomethylene]decane in 5 ml of dichloromethane solution and cool to - At 78°C, carefully add 3mmol of liquid bromine, and react at this temperature for 2 hours. After rising to room temperature, add 5ml of saturated sodium sulfite aqueous solution and stir for 15 minutes. Wash twice with 10 ml of ether, combine the organic layers, wash with water, and dry over anhydrous magnesium sulfate. After filtering off the desiccant, the organic solution was distilled off under reduced pressure to remove the solvent, and then separated by silica gel column chromatography, using petroleum ether as the eluent. The pure product (5Z, 6Z)-5,6-bis(dibromomethylene)decane 0.440 g (purity>98%, colorless liquid) can be obtained with an isolated yield of 92%. The NMR and...
Embodiment 2
[0029] The second of class I compounds in the structural formula (R 1 =R 2 =n-Bu): Synthesis of (5Z,6Z)-5,6-bis(iodobromomethylene)decane
[0030] In a 25 mL round bottom flask, dissolve 1 mmol of (5Z,6Z)-5,6-bis[1-iodo-1-(trimethylsilyl)methylidene]decane in 8 mL of dichloromethane solution, cooled to -78°C, carefully added 2.5mmol of liquid bromine, and reacted at this temperature for 2 hours, after rising to room temperature, added 5ml of saturated aqueous sodium sulfite solution and stirred for 15 minutes, added 5ml of dichloromethane to the mixed solution and divided solution, and the aqueous layer was washed twice with 10 ml of petroleum ether. The organic layers were combined, washed with water, and dried over anhydrous sodium sulfate. After filtering off the desiccant, the organic solution was distilled off under reduced pressure to remove the solvent, and then separated by silica gel column chromatography, using petroleum ether as the eluent. 0.510 g of pure produ...
Embodiment 3
[0032] Three of I class compounds in the structural formula (R 1 =R 2 =n-Bu): Synthesis of (Z)-5-(iodobromomethylene)-6-(dibromomethylene)decane
[0033]In a 25 ml round bottom flask, 1 mmol of (5Z, 6Z)-5-[1-iodo-1-(trimethylsilyl)methylidene]-6-[1-bromo-1-( Dissolve trimethylsilyl) methylene] decane in 10 ml of dichloromethane solution, cool to -78°C, carefully add 3.5 mmol liquid bromine, and react at this temperature for 3 hours, then add 5 ml after rising to room temperature Saturated sodium sulfite aqueous solution was stirred for 10 minutes, 5 ml of dichloromethane was added to the mixture, and the layers were separated. The aqueous layer was washed twice with 10 ml of n-pentane. The organic layers were combined, washed with water, and dried over anhydrous magnesium sulfate. After filtering off the desiccant, the organic solution was distilled off under normal pressure to remove the solvent, and then separated by silica gel column chromatography, using petroleum ether ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com