Electro-catalyst of zinc-air battery and method for making same
A zinc-air battery and electrocatalyst technology, which is applied in the direction of catalyst activation/preparation, battery electrodes, chemical instruments and methods, etc., can solve the problems of inability to meet the needs of high-power electrical appliances, poor chemical stability of metal Co elements, and discharge current of zinc-air batteries. Low-level problems, to achieve the effect of abundant reserves, short heat treatment time and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method for an electrocatalyst for a zinc-air battery, comprising the steps of:
[0030] In the first step, LiNO was weighed according to the stoichiometric molar ratio Li:Ni:Mn=1.00:0.20:1.80 3 , Ni(CH 3 COO) 2 4H 2 O and 50 wt% Mn(NO 3 ) 2 solution, distilled water was added to form a saturated aqueous solution.
[0031] In the second step, citric acid and ethylene glycol are added to the above reaction system, and the pH of the reaction system is adjusted to 5.0 with ammonia water. Wherein, the molar ratio of citric acid:ethylene glycol:total metal ions is 3.0:4.0:1.0.
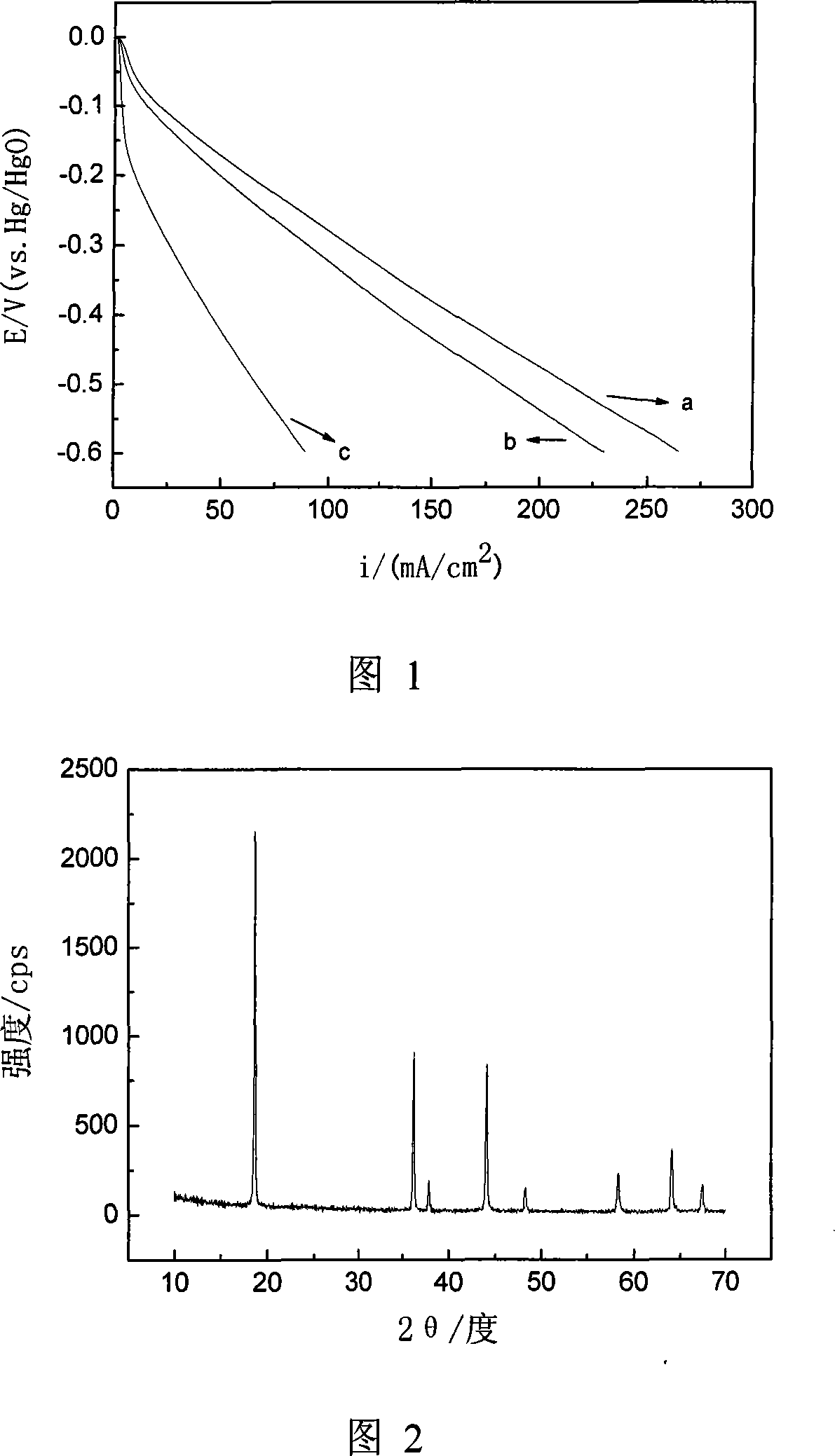
[0032] The third step is to heat the reaction system in a constant temperature water bath at 80°C to gradually evaporate the water to obtain a transparent sol, continue to dehydrate for 4 hours to form a wet gel, and then heat and dry the wet gel at 120°C for 12 hours to obtain Honeycomb-shaped xerogel, and finally heat-treat the xerogel at 500°C for 6 hours in an air atmosphere, ...
Embodiment 2
[0037] A preparation method for an electrocatalyst for a zinc-air battery, comprising the steps of:
[0038] In the first step, LiNO was weighed according to the stoichiometric molar ratio Li:Ni:Mn=1.00:0.10:1.90 3 , Ni(CH 3 COO) 2 4H 2 O and 50 wt% Mn(NO 3 ) 2 solution, distilled water was added to form a saturated aqueous solution.
[0039] In the second step, citric acid and ethylene glycol are added to the above reaction system, and the pH of the reaction system is adjusted to 5.0 with ammonia water. Wherein, the molar ratio of citric acid:ethylene glycol:total metal ions is 3.0:4.0:1.0.
[0040] The third step is to heat the reaction system in a constant temperature water bath at 80°C to gradually evaporate the water to obtain a transparent sol, continue to dehydrate for 3 hours to form a wet gel, and then heat and dry the wet gel at 120°C for 12 hours to obtain Honeycomb-shaped xerogel, and finally heat-treat the xerogel at 500°C for 6 hours in an air atmosphere, ...
Embodiment 3
[0044] A preparation method for an electrocatalyst for a zinc-air battery, comprising the steps of:
[0045] In the first step, LiNO was weighed according to the stoichiometric molar ratio Li:Ni:Mn=1.00:0.50:1.50 3 , NiC 2 o 4 2H 2 O and 50 wt% Mn(NO 3 ) 2 solution, distilled water was added to form a saturated aqueous solution.
[0046] In the second step, citric acid and ethylene glycol are added to the above reaction system, and the pH of the reaction system is adjusted to 5.5 with ammonia water. Wherein, the molar ratio of citric acid:ethylene glycol:total metal ions is 2.0:3.5:1.0.
[0047] The third step is to heat the reaction system in a constant temperature water bath at 90°C to gradually evaporate the water to obtain a transparent sol, continue to dehydrate for 2 hours to form a wet gel, and then heat and dry the wet gel at 100°C for 16 hours to obtain Honeycomb-shaped xerogel, and finally heat-treat the xerogel at 900°C for 1 hour in an air atmosphere, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com