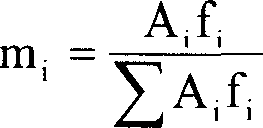Preparation method of cyclohexanone oxime
A technology of cyclohexanone oxime and cyclohexanone, which is applied in the field of preparation of cyclohexanone oxime, can solve the problems of reduced effective utilization rate of catalyst, complex process, easy adhesion, etc., achieve high solid-liquid separation efficiency, improve reaction efficiency, The effect of increasing the heat transfer rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
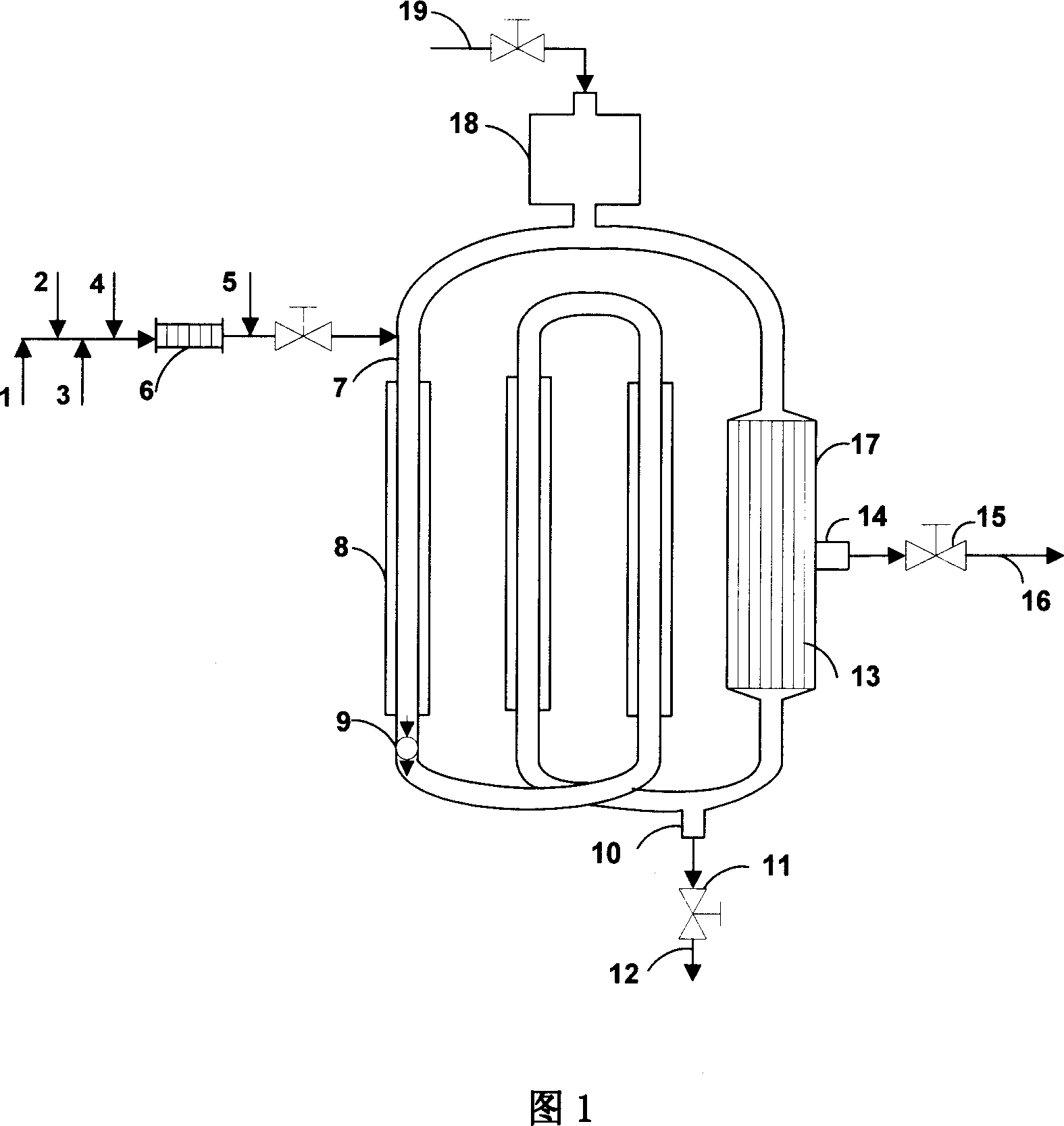
[0060] See Figure 1 for the reaction process. The effective volume of the reactor is 5 liters, the separation element is an inorganic ceramic membrane tube with 19 channels φ4, and the effective filtration area is 0.2m 2 , The slurry is forced to circulate by a circulation pump, the reaction raw materials and reaction products are continuously fed into and out of the reactor, the catalyst is added at one time, and the heat exchange through the jacket is used to maintain isothermal operation.
[0061] Concrete feed parameter, separation system parameter and reaction result are as follows:
[0062] Cyclohexanone = 620 g / h
[0063] Tert-butanol (about 15w% water) = 3000 g / h
[0064] 27.5w% hydrogen peroxide = 860 g / hour
[0065] Liquid ammonia = 180 g / h
[0066] Reaction temperature = 83±1°C
[0067] Reaction pressure = 0.3MPa (gauge pressure)
[0068] Slurry linear velocity in the ring pipe = 2 m / s
[0069] Catalyst concentration = 3% by weight
[0070] The average resid...
Embodiment 2
[0081] The reaction process is shown in Figure 1, and the specific feed parameters, separation system parameters and reaction results are as follows:
[0082] Cyclohexanone = 750 g / h
[0083] Tert-butanol (about 15w% water) = 3500 g / h
[0084] 27.5w% hydrogen peroxide = 995 g / hour
[0085] Liquid ammonia = 170 g / h
[0086] Reaction temperature = 76±1°C
[0087] Reaction pressure = 0.4MPa (gauge pressure)
[0088] Slurry linear velocity in the ring pipe = 2 m / s
[0089] Catalyst concentration = 2% by weight
[0090] The average residence time of the material in the reactor = 50 minutes
[0091] Filtration system recoil cycle = 30 minutes
[0092] Filtration system recoil time = 2 seconds
[0093] Its reaction result is as follows:
[0094] Cyclohexanone conversion = 97.3%
[0095] Hydrogen peroxide conversion rate = 98.6%
[0096] Reaction selectivity in terms of cyclohexanone = 99.1%
[0097] Effective utilization rate of hydrogen peroxide = 90.6%
[0098] Cyclohexa...
Embodiment 3
[0101] The reaction process is shown in Figure 1, and the specific feed parameters, separation system parameters and reaction results are as follows:
[0102] Cyclohexanone = 500 g / h
[0103] Tert-butanol (about 15w% water) = 2400 g / h
[0104] 27.5w% hydrogen peroxide = 725g / hour
[0105] Liquid ammonia = 130 g / h
[0106] Reaction temperature = 89±1°C
[0107] Reaction pressure = 0.2MPa (gauge pressure)
[0108] Slurry linear velocity in the ring pipe = 2 m / s
[0109] Catalyst concentration = 4% by weight
[0110] The average residence time of the material in the reactor = 78 minutes
[0111] Filtration system recoil cycle = 20 minutes
[0112] Filtration system recoil time = 2 seconds
[0113] Its reaction result is as follows:
[0114] Cyclohexanone conversion = 99.4%
[0115] Hydrogen peroxide conversion rate = 100%
[0116] Reaction selectivity in terms of cyclohexanone = 98.6%
[0117] Effective utilization rate of hydrogen peroxide = 87.0%
[0118] Cyclohexa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com