Method of producing C8 arene isomerization catalyst
A technology for isomerization and catalyst of aromatic hydrocarbons, which is applied in the field of preparation of catalysts for isomerization of C8 aromatic hydrocarbons, can solve the problems of high catalyst production cost, high price, and limitation of large-scale production and application of acidic components, and achieve high selectivity and synthesis Effects of cost reduction and low loss of aromatics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1~5
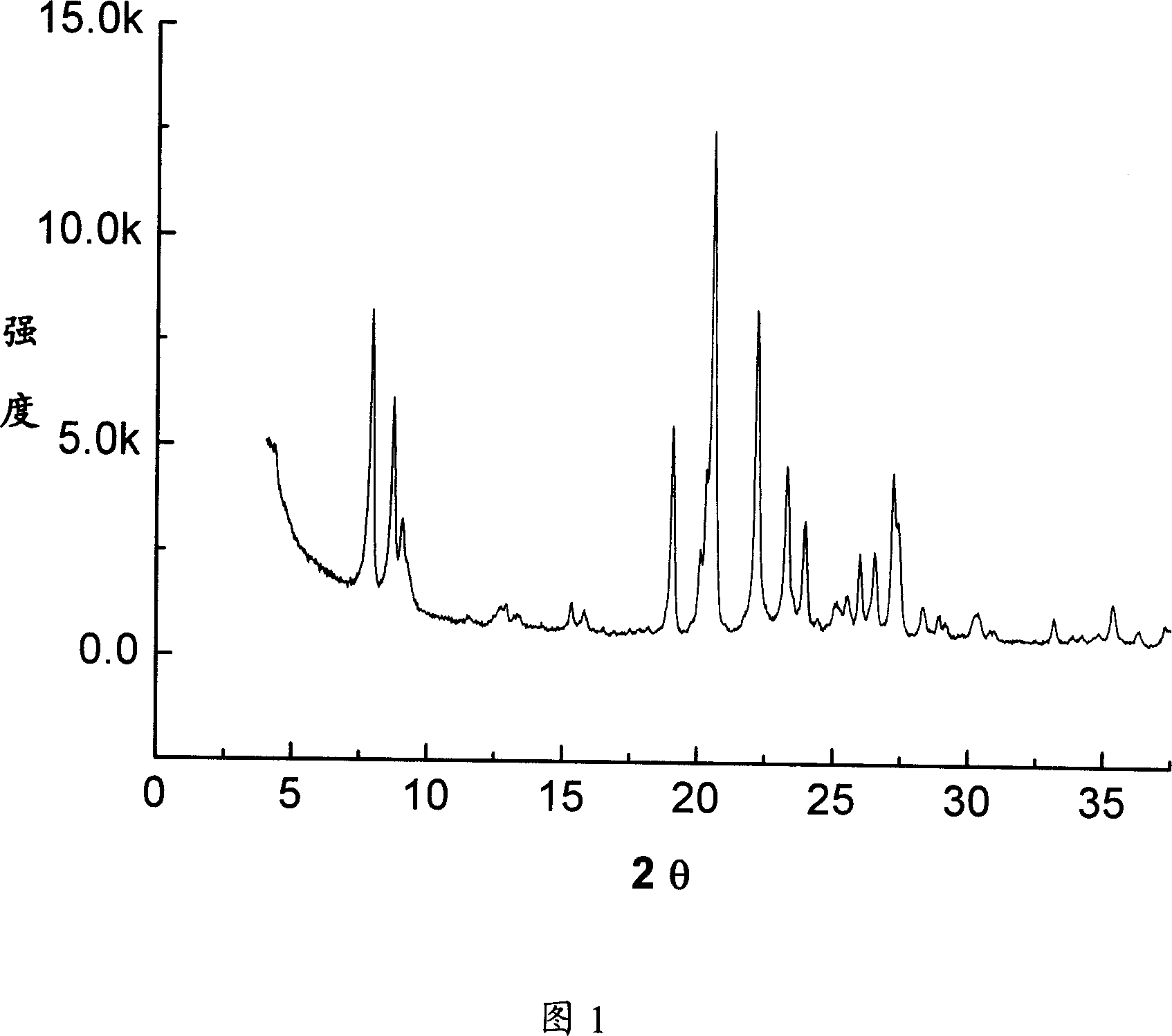
[0057] The following examples use the method of the present invention to prepare EUO type molecular sieves.
[0058] (1) Preparation of template precursor
[0059] In the four-neck flask equipped with reflux condenser, stirrer, thermometer and dropping funnel, first add 100g of hexanediol (Beijing Chemical Reagent Company, chemically pure, content ≥ 99% by mass) and 75g of hydrogen bromide ( Sinopharm Group Chemical Reagent Co., Ltd., analytically pure, content ≥ 99% by mass), stirred at 25°C to fully dissolve the two, heated to 40°C, and slowly added 100g of concentrated sulfuric acid (Beijing Chemical Plant, chemically pure, 95 ~98% by mass), then add 200g of hydrogen bromide, slowly heat up to reflux temperature and react for 4 hours, wash the oil phase with an equal volume of water and 5% by mass of sodium hydroxide solution to make it neutral, and obtain the crude dihydrogen bromide Hexyl bromide, which contains 85% by mass of dibromohexane, 13% by mass of monobromohexan...
example 6
[0067] The following example prepares C8 Aromatic isomerization catalyst.
[0068] (1) kneading molding: get the molecular sieve D prepared by 45.00 grams of example 1 1 , 45.94 grams of pseudo-boehmite powder (produced by Wenzhou Catalyst Factory, Al 2 o 3 The content is 76.3% by mass), 5 grams of scallop powder and 30 milliliters of dilute nitric acid solution of 2% by volume are mixed evenly, and kneaded to make strip-shaped particles.
[0069] (2) Ion exchange: Take 50 g of the particles prepared in step (1) and 250 ml of 5 mass % ammonium chloride aqueous solution at 90° C. for 2 hours under continuous stirring, and exchange 2 times in total. The sodium content in the carrier after ion exchange was 0.04% by mass. The particles after ion exchange were dried at 110° C. for 12 hours, and calcined at 550° C. for 4 hours to obtain a carrier.
[0070] (3) loaded metal elements: get 30 grams of the carrier obtained after the ion exchange in step (2), add it to 50 milliliters...
example 7
[0073] Get the EUO type molecular sieve that 33.50 grams example 2 prepares, the hydrated aluminum oxide of 66.42 grams (Beijing Chemical Reagent No. 2 factory, content 65 mass %), 5 grams of silica gel (Shenyang Chemical Co., Ltd. production), 40 milliliters 3 volume % dilute nitric acid The solution is mixed evenly and kneaded to form strip-shaped particles.
[0074] Get 50 grams of strip-shaped particles according to the method of Example 6 (2) step, perform ion exchange, dry, and roast to obtain a carrier, and the sodium content in the carrier is 0.045% by mass. Prepare catalyst E by the method of (3) step 2 , the difference is that the carrier is added to 65 ml of palladium nitrate solution with a concentration of 4.38 mg / ml and immersed for 44 hours at 22 ° C, and the catalyst E obtained after drying and roasting 2 The composition and platinum dispersion are shown in Table 2.
[0075] The granules prepared in step (3) were heated at 400°C and under normal pressure with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com