Synthetic method of carbostyrile kind antibiotic multi cluster antigen
A technology of multi-cluster antigens and quinolones, which is applied in the field of biochemical industry to achieve the effect of simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
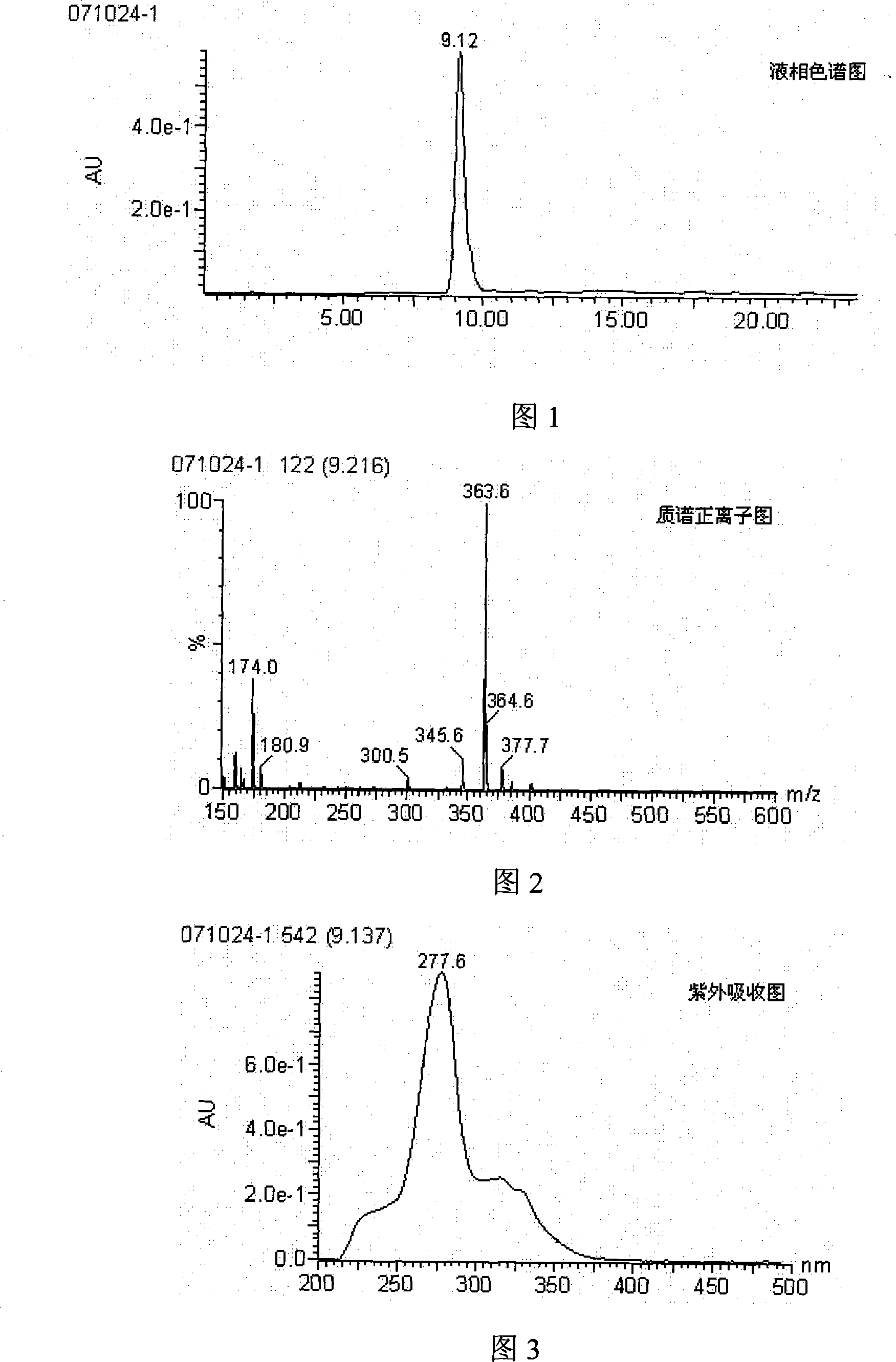
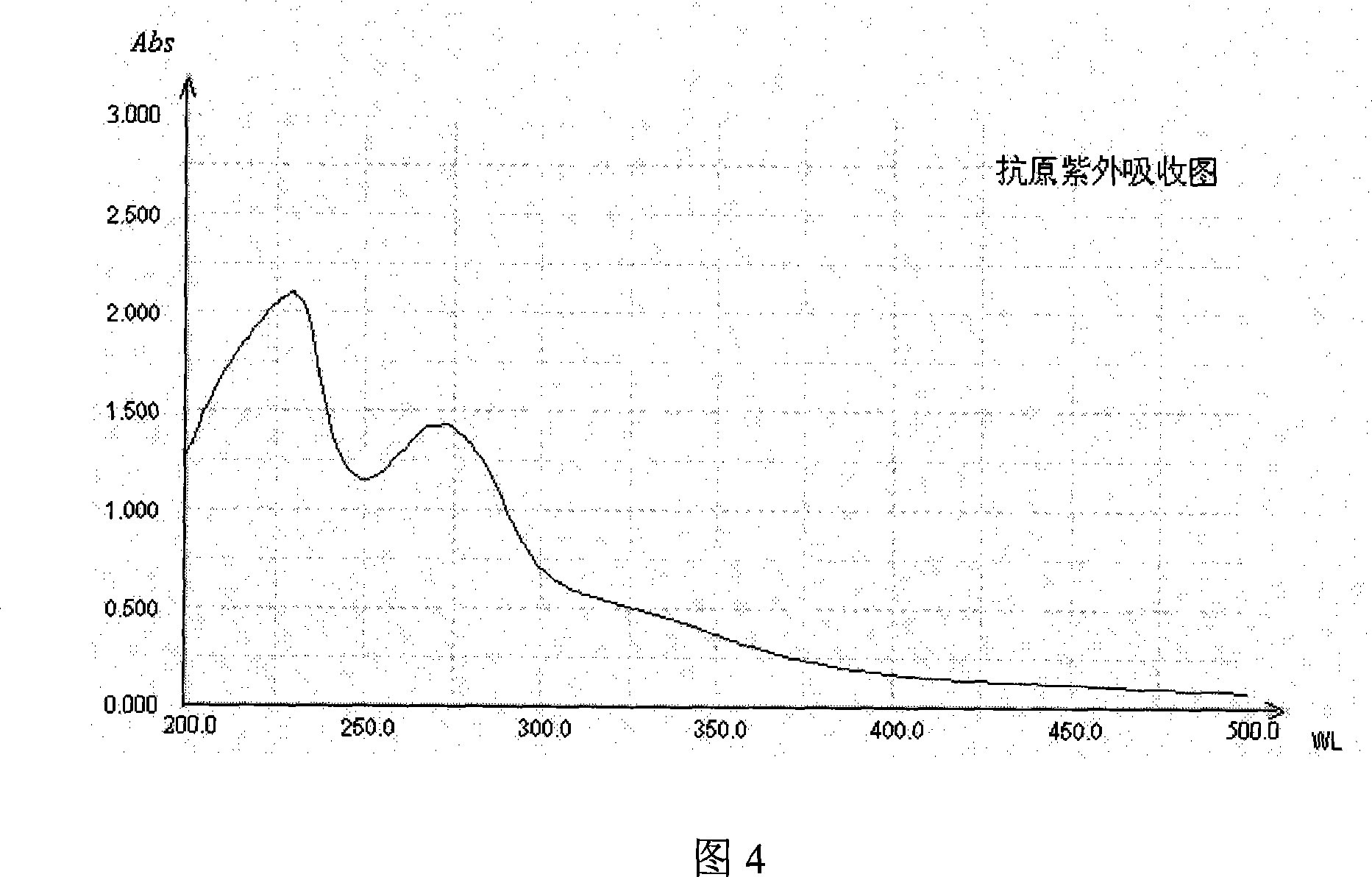
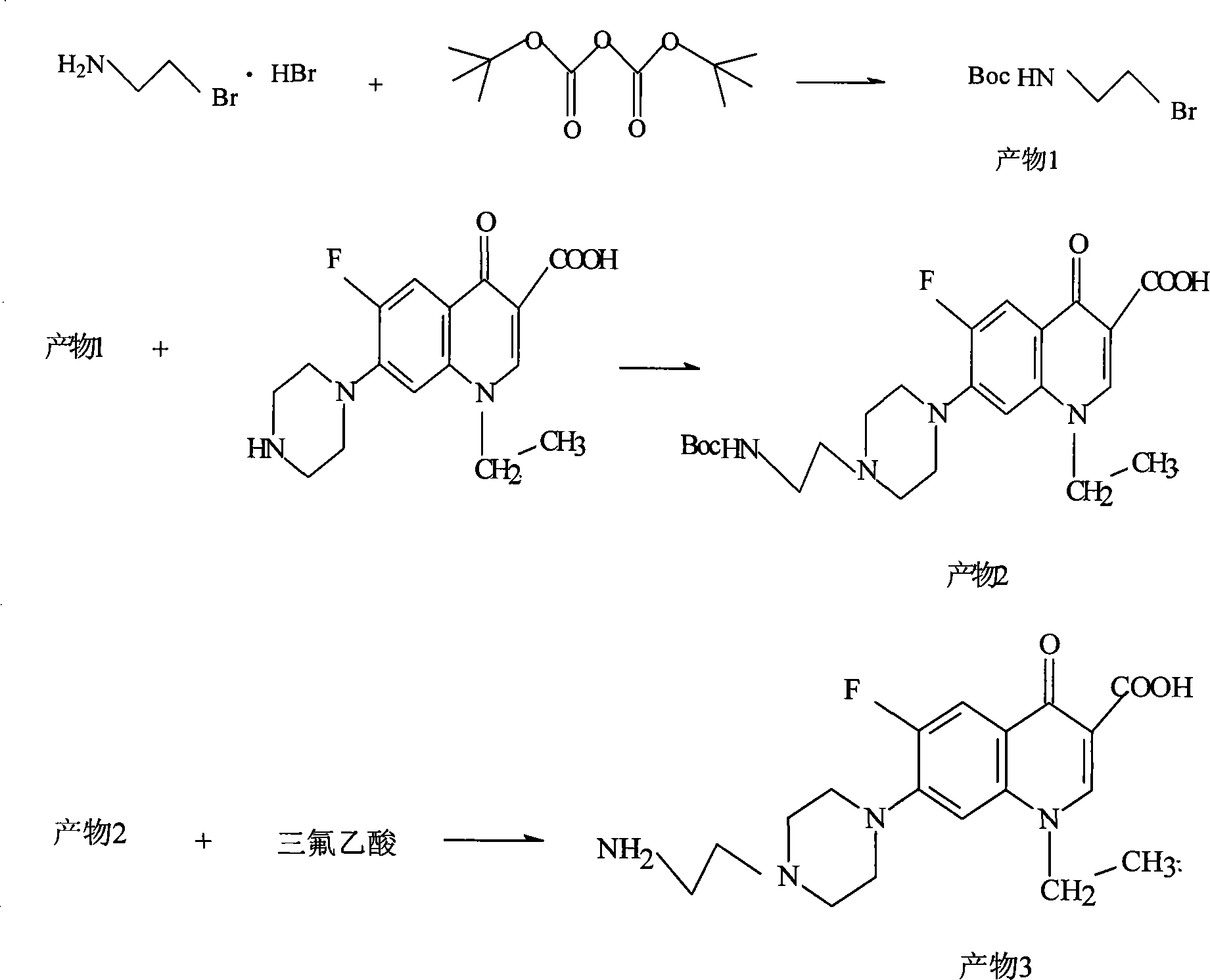
[0030] (1) Synthesis of artificial hapten
[0031] Synthesis:
[0032] Synthesis of product 1:
[0033] In a 1L round bottom flask, add 500mL of methanol, 2-bromoethylamine hydrobromide (10g, 49mmol), triethylamine (30mL), di-tert-butyl carbonate Boc 2 O (13g, 60mmol), stirred, heated to 60°C, reacted for 1h, then reacted overnight at room temperature. The reaction solution was concentrated in vacuo, the residue was dissolved in 500mL of dichloromethane, washed with 0.5N hydrochloric acid (250mL×2), saturated saline (250mL), saturated NaHCO 3 (250 mL), dried over anhydrous magnesium sulfate, filtered, and concentrated to give product 1 as a light yellow liquid (yield 63%), which was directly used in the next reaction.
[0034] Synthesis of product 2:
[0035] In a 250ml round bottom flask, add norfloxacin (5.499g, 17.2mmol), DMF (70mL), stir well, add triethylamine (4.8mL, 35mmol), then add product 1 (4.631g, 20.6mmol ), heated up to 80°C, and reacted overnight. Cool the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com