Recombinant octreotide and uses thereof
A technology of octreotide and nucleotide sequence, which is applied in the field of recombinant octreotide and its application, can solve the problems that can not be used to prepare drugs for targeted therapy, can not realize targeted brachytherapy of tumor cells, etc., and achieve fast blood clearance, Reagents are cheap and easy to obtain, and the method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Preparation of recombinant Tyr-Octreotide
[0028] According to the provided sequence SEQ ID NO.2, it is well known to those skilled in the art to synthesize small molecule polypeptides by using known techniques. In the present invention, Tyr-Octreotide, which replaces phenylalanine with tyrosine, was researched by Beijing Huada Gene Prepared in the center, the prepared small molecular protein Tyr-Octreotide was analyzed by high-performance liquid chromatography (HPLC), and after molecular weight determination, it was the desired protein with a purity >95%. Sequencing analysis is SEQ ID NO.2.
Embodiment 2
[0029] Embodiment 2: preparation 131 I-Tyr-Octreotide
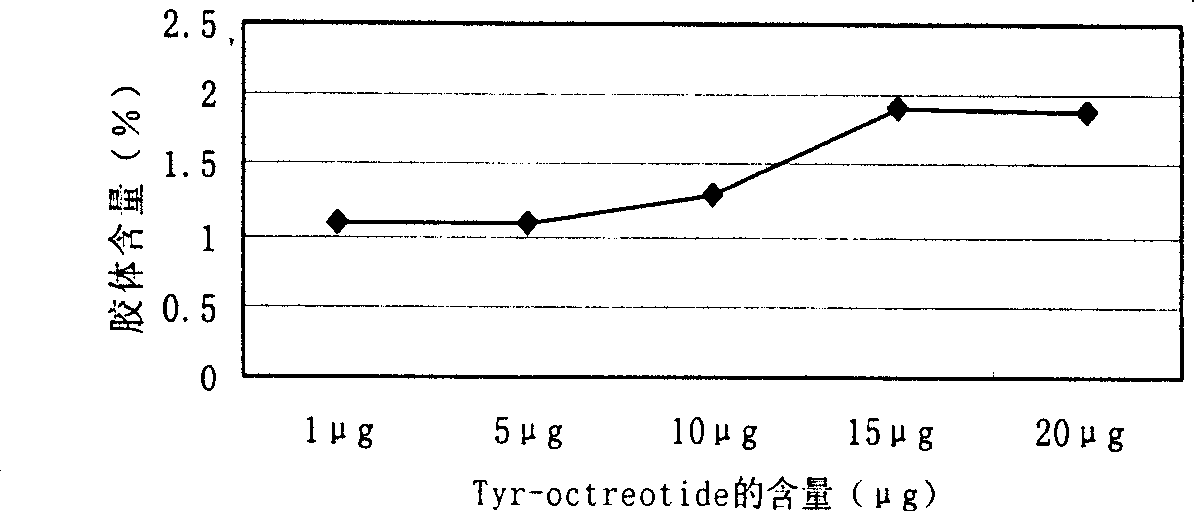
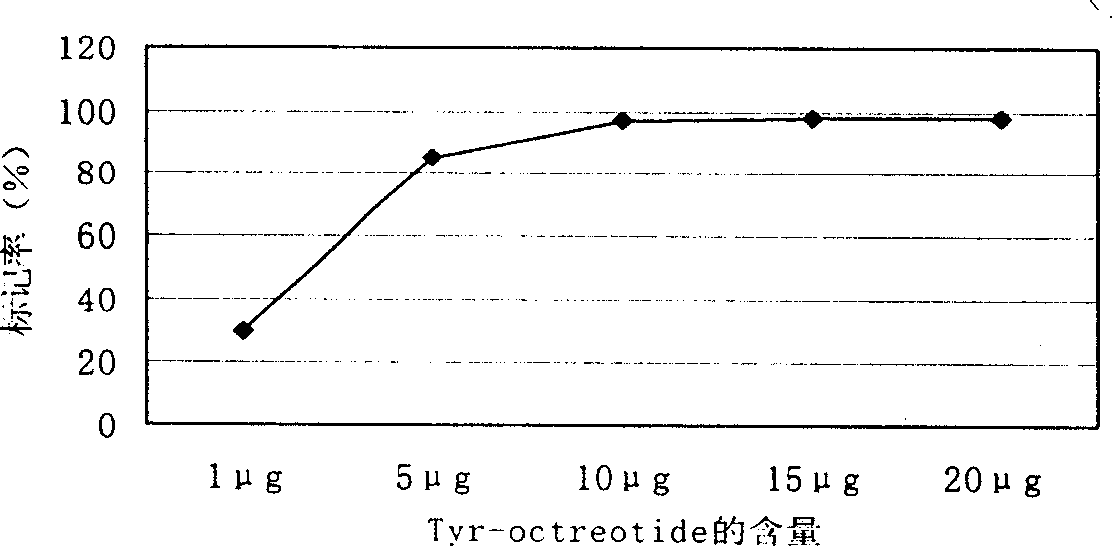
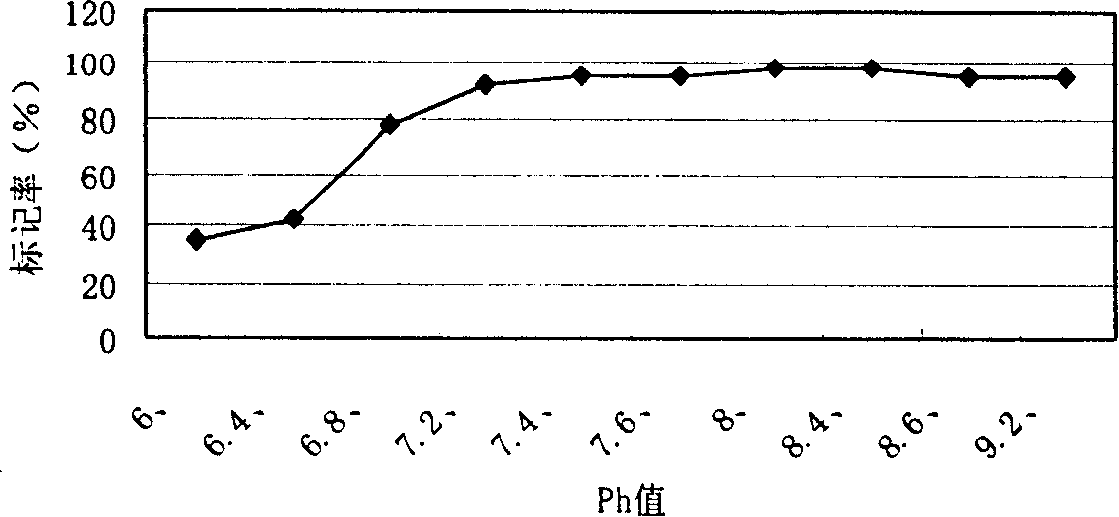
[0030] 131 Labeling of I-Tyr-Octreotide: take 0.2mol / L NaH 2 PO 4 and 0.2mol / L Na 2 HPO 4 Configured as 0.2mol / L PB; weigh 0.3g of Ch-T and Na 2 S 2 o 5 Prepare a reaction solution of 30 μg / μL; prepare Tyr-Octreotide with a concentration of 1 mg / ml. Take 150 μL of PB at 25°C, add Octreotide 10 μL, Na 131 I (0.15mCi) 0.1ml, Ch-T 4μL reaction 3min and shake quickly to mix, then add Na 2 S 2 o 5 5 μL, labeling conditions: ①The pH value of phosphate buffer is from 6.0 to 9.2; ②The amount of Tyr-Octreotide is 1 μg, 5 μg, 10 μg, 15 μg, 20 μg; ③Reaction temperature is 25°C, ④Reaction time is 2-3min. mark in minutes 131 I-Tyr-Octreotide.
[0031] Determination of prepared 131 The radiochemical purity and in vitro stability of I-Tyr-Octreotide: Two systems are used: System 1 uses Xinhua No. 1 chromatography paper as the stationary phase, and normal saline as the developer. 131 I-Tyr-Octreotide and colloid ratio shi...
Embodiment 3
[0033] Example 3: 131 Distribution of I-Tyr-Octreotide in normal mouse tissues
[0034] Thirty Kunming mice (20-25g) were randomly divided into 6 groups, 5 in each group. tail vein injection 131 I-Tyr-Octreotide 0.15mL (3.7MBq), imaging was carried out at 30min, 4, 8, 12, 24, 48h after administration, and then the mice were killed by taking blood from the eyeballs. Take important tissues and organs, including heart, liver, spleen, lung, kidney, stomach, small intestine (1cm away from the stomach), large intestine, pancreas, femur, muscle, brain, thyroid, etc. 13 tissues and organs, weigh and measure the radioactive count, calculate %ID / g (radioactive count per gram of tissue / total radioactive count injected into mice × 100%). The blood clearance curve was drawn with time as the abscissa and radioactive count as the ordinate, and was fitted with Origin5.0 software to calculate and analyze its pharmacokinetic parameters in normal mice.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com