Compound and organic light-emitting element
A compound, unsubstituted technology, applied in the field of light-emitting devices of organic compounds, can solve problems such as degradation, no specific description of organic compounds, time-determined changes, etc., and achieve the effect of excellent durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145]
[0146]
[0147] 698 mg (1.5 mmol) of compound 1 and 576 mg (1.5 mmol) of compound 2 were prepared. These compounds and 15 ml of toluene, 7.5 ml of ethanol, 5 ml of a 2M aqueous solution of sodium carbonate and 100 mg (0.09 mmol) of tetrakis (triphenylphosphine) palladium (0) were charged into a 100 ml flask, and the whole was heated at 80 °C under nitrogen atmosphere and stirred for 8 hours. After the reaction was completed, the reaction liquid was extracted with toluene, and then the organic layer was washed with water, dried over magnesium sulfate, and evaporated to dryness under reduced pressure. After purification by silica gel column chromatography (eluent: toluene), it is recrystallized from toluene / ethanol. The resulting crystals were vacuum-dried and then purified by sublimation to yield 570 mg of Exemplary Compound No. A-85 (yield: 59.1%).
[0148] Compound M+ of 642.3 was confirmed by matrix-assisted laser desorption / ionization-time-of-flight mass spe...
Embodiment 2
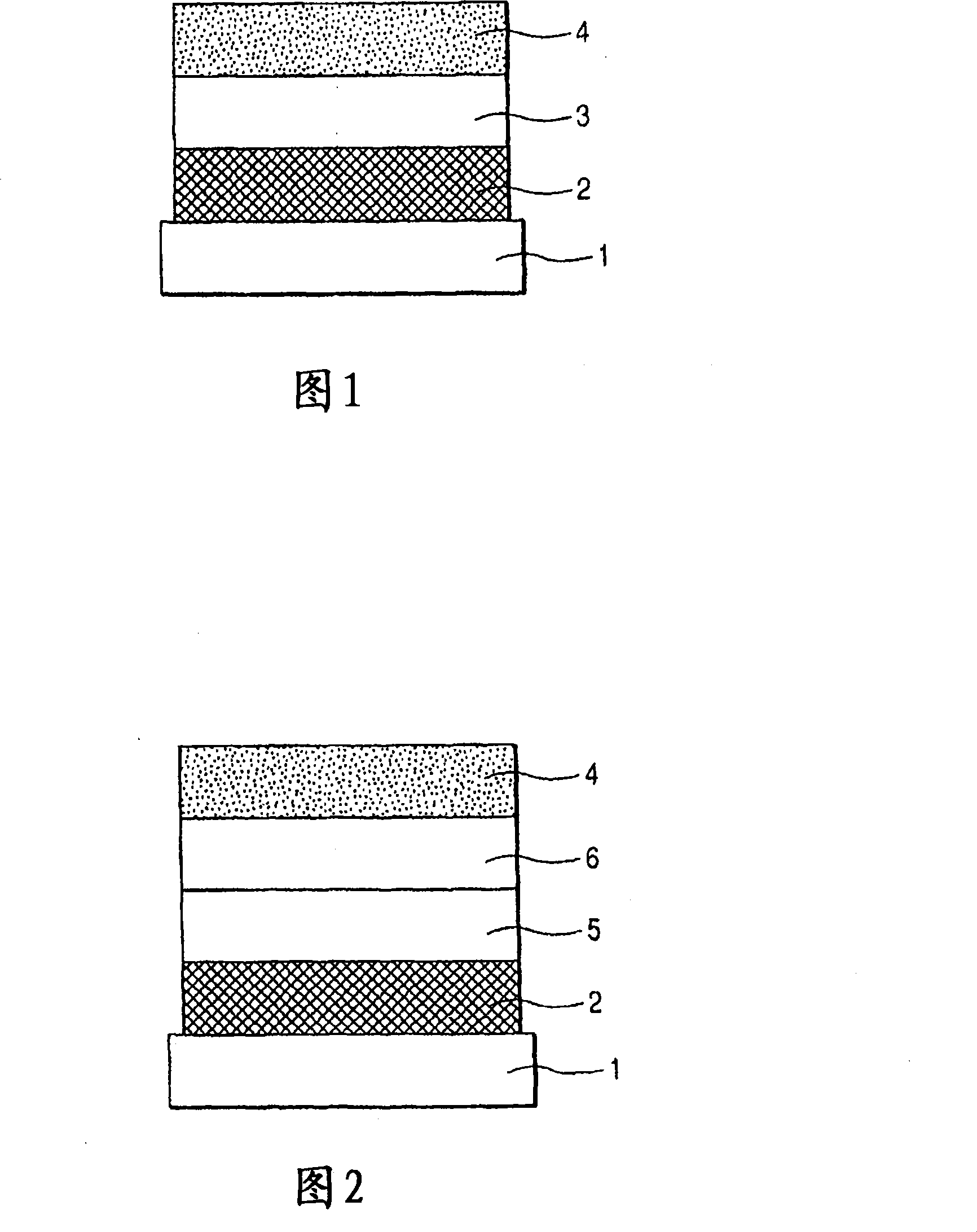
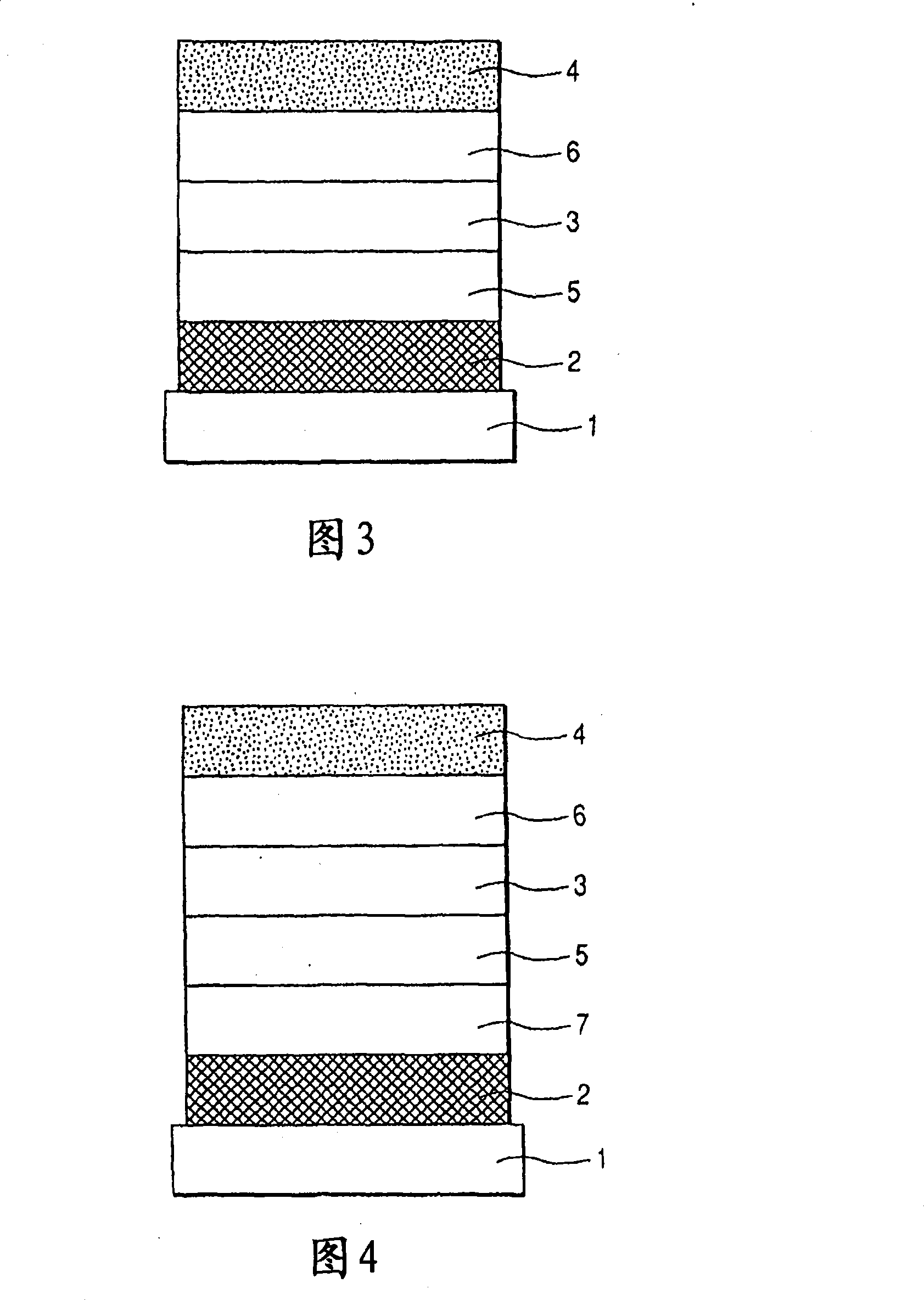
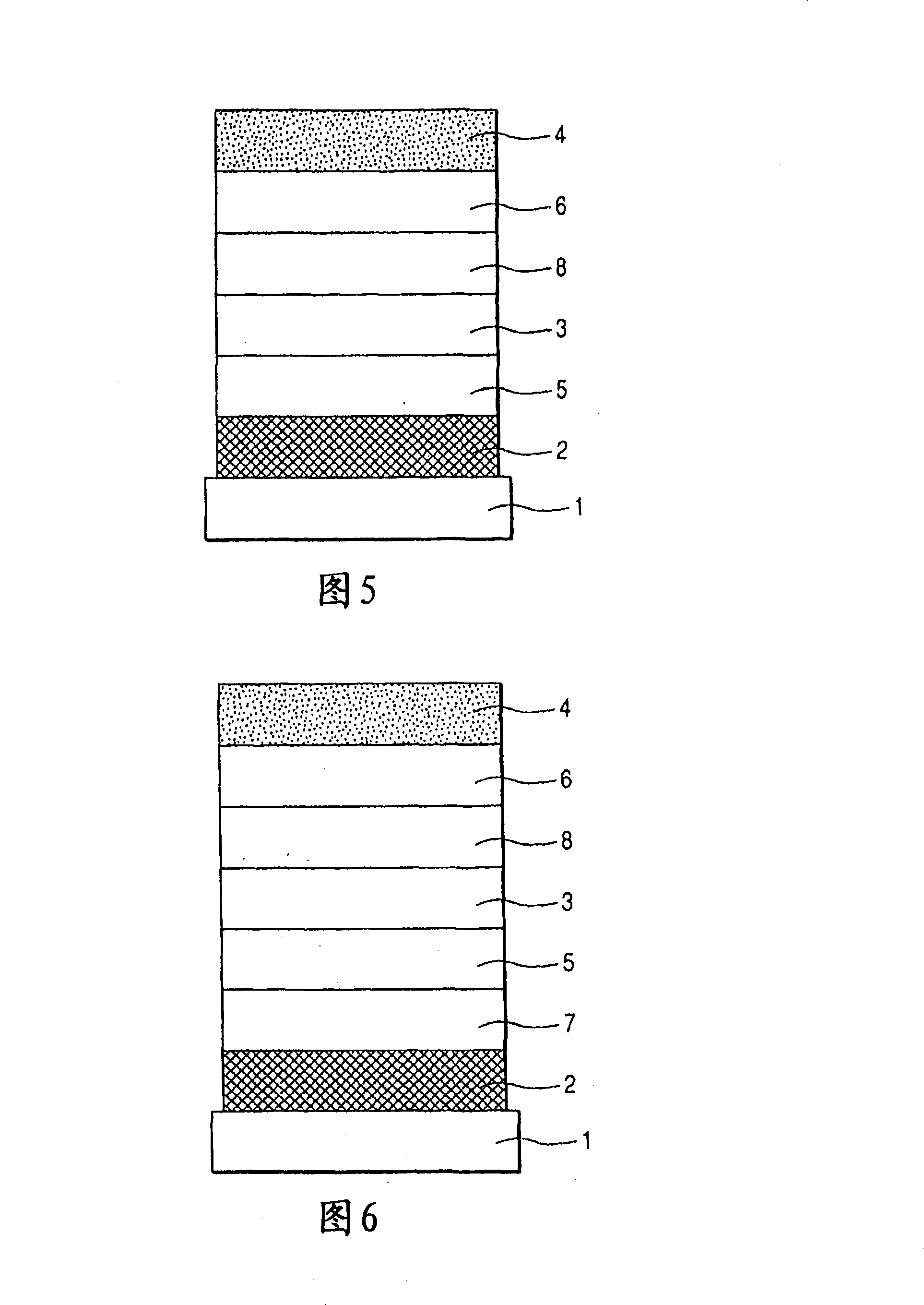
[0153] A transparent conductive support substrate having a film of indium tin oxide (ITO) having a thickness of 120 nm as an anode formed on a glass substrate 1 by a sputtering method was prepared. The transparent conductive support substrates were ultrasonically cleaned sequentially with acetone and isopropanol (IPA), followed by boiling IPA, then dried and further cleaned with UV / ozone, and used.
[0154] Then, on the transparent conductive supporting substrate, a chloroform solution of Compound 3 represented by the following structural formula was coated with a film thickness of 20 nm by spin coating to form a hole transport layer.
[0155] In addition, having 10 -5 Using resistance heating in a vacuum chamber at an internal pressure of Pa, the following organic layers and electrode layers were sequentially formed by vacuum vapor deposition to fabricate a device.
[0156] Light-emitting layer (20 nm): Exemplary Compound No. A-85 and Compound 4 (10% by weight concentration)...
Embodiment 3
[0164]
[0165]
[0166] 782 mg (1.5 mmol) of compound 5 and 576 mg (1.5 mmol) of compound 2 were prepared. These compounds and 15 ml of toluene, 7.5 ml of ethanol, 5 ml of a 2M aqueous solution of sodium carbonate, and 100 mg (0.09 mmol) of tetrakis(triphenylphosphine) palladium (0) were charged into a 100 ml flask, and the whole was heated at 80° C. Stir under nitrogen atmosphere for 8 hours. After the reaction was completed, the reaction liquid was extracted with toluene, and then the organic layer was washed with water, dried over magnesium sulfate, and evaporated to dryness under reduced pressure. After purification by silica gel column chromatography (eluent: toluene), it is recrystallized from toluene / ethanol. The resulting crystals were vacuum-dried and then purified by sublimation to yield 710 mg of Exemplary Compound No. A-91 (yield: 67.7%).
[0167] Compound M+ of 698.4 was confirmed by matrix-assisted laser desorption / ionization-time-of-flight mass spectrome...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com