Sustained-release injection containing angiogenic inhibitor
A technology of vascular inhibitors and sustained-release injections, which is applied in the field of anti-cancer sustained-release injections, and can solve problems such as increased resistance to anti-cancer drugs and treatment failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
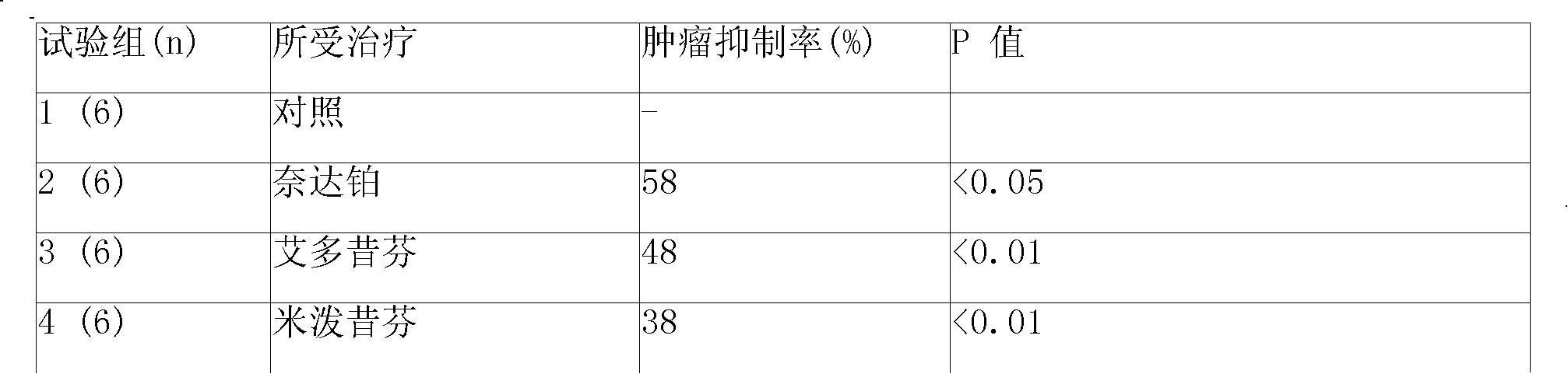
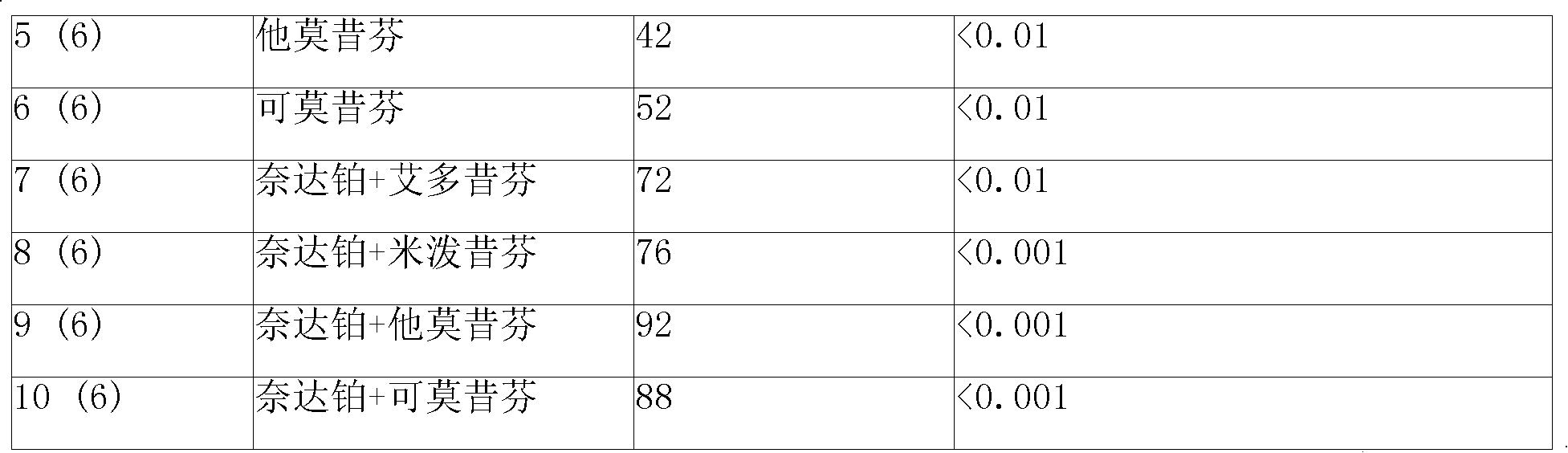
[0158] Put 80mg of polyphenylpropane (p-carboxyphenylpropane (p-CPP): sebacic acid (SA) at 20:80) copolymer into a container, add 100ml of dichloromethane, dissolve and mix well, then add 10mg of heptane Platinum and leuprolide, after re-shaking, microspheres for injection containing 10% heptaplatin and 10% leuprolide were prepared by spray-drying method. Then suspend the microspheres in physiological saline containing 15% mannitol to prepare the corresponding suspension-type sustained-release injection with a viscosity of 20cp-300cp (at 20°C-30°C). The drug release time of the slow-release injection in physiological saline in vitro is 10-15 days, and the drug release time in mice subcutaneous is about 20-30 days.
Embodiment 2
[0160] The method step of being processed into sustained-release injection is the same as in Example 1, but the difference is that the contained anticancer active ingredients and their weight percentages are:
[0161] (a) 5-20% cisplatin, carboplatin, omaplatin, dexomaplatin, heptaplatin, lobaplatin, nedaplatin or oxaliplatin;
[0162] (b) 5-30% triptorelin, goserelin, leuprolide, anastrozole, edoxifene, milprexifen, tamoxifen, 4-monohydroxytamoxifen , comoxifen, raloxifene, steroid estrogen, anticancer stenol, 4-hydroxytamoxifen, flutamide, aminoglutethimide, pirumide, megestrol, methyl hydroxyprogesterone, clomiphene, toremifene, letrozole, anastrozole, exemestane, or bicalutamide; or
[0163] (c) 5-20% cisplatin, carboplatin, omaplatin, dextro-omaplatin, heptaplatin, lobaplatin, nedaplatin or oxaliplatin and 5-30% triptorelin, goserelin , leuprolide, anastrozole, edoxifene, milprexifen, tamoxifen, 4-monohydroxytamoxifen, comoxifen, raloxifene, steroid antiestrogens, antic...
Embodiment 3
[0166] Put 70 mg of polylactic acid (PLGA, 75:25) with a peak molecular weight of 25,000 into a container, add 100 ml of dichloromethane, dissolve and mix well, add 15 mg of lobaplatin and 15 mg of gefitinib, re-shake and dry in vacuo Remove organic solvents. The dried drug-containing solid composition was frozen and pulverized to make micropowder containing 15% lobaplatin and 15% gefitinib, and then suspended in physiological saline containing 1.5% carboxymethylcellulose sodium to prepare the corresponding mixed Suspension-type sustained-release injection with a viscosity of 220cp-340cp (at 20°C-30°C). The drug release time of the slow-release injection in physiological saline in vitro is 10-15 days, and the drug release time in mice subcutaneous is about 20-30 days.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com