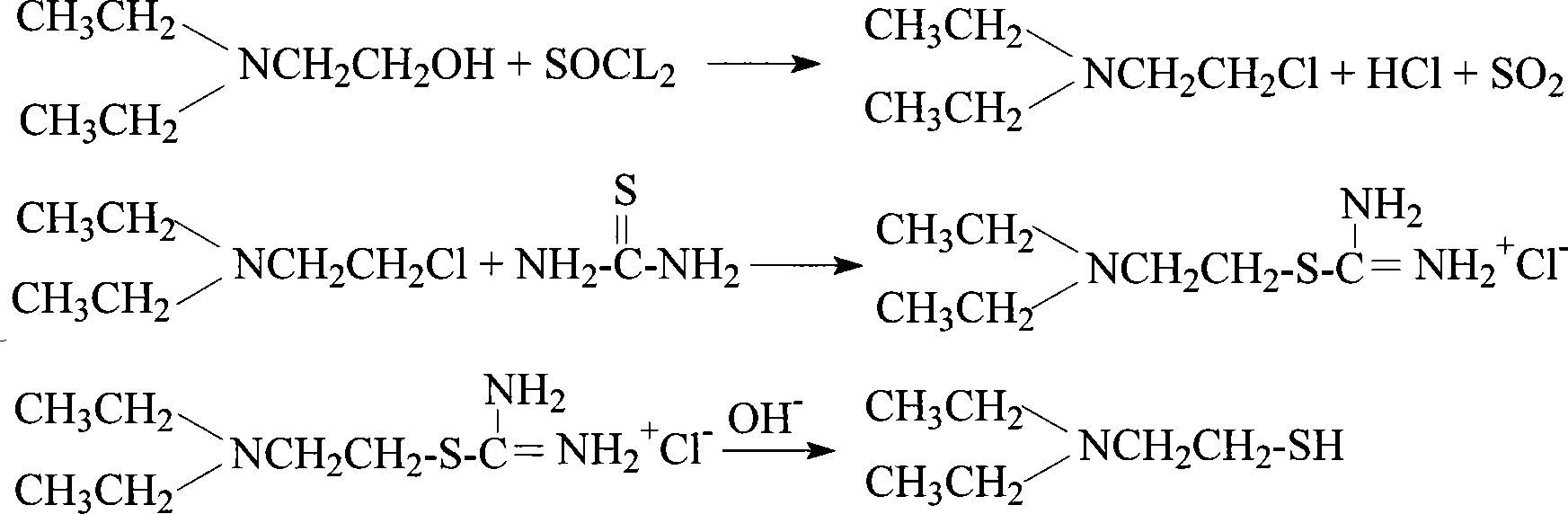
Method for preparing diethylamino ethanethiol
A technology of diethylaminoethanethiol and ethylaminoethanethiol, which is applied in the field of medicine, can solve the problems of consuming a large amount of organic solvents or water resources, the smell of sodium hydrosulfide is unpleasant, and it is not suitable for industrial applications, so as to reduce production costs , easy layering, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 110 mol of thionyl chloride to 150 mol of benzene, and then add 100 mol of diethylaminoethanol dropwise to the above solution at a temperature of 35 to 40°C under external cooling conditions to form a reaction mixture. ℃ reflux reaction for 2h, then the reaction mixture is cooled to below 40 ℃, and 400mol water is added dropwise, then the reaction mixture is cooled to below 20 ℃, and the pH value of the reaction mixture is adjusted with a mass percent concentration of 50% sodium hydroxide solution 10. The reaction mixture was left to stand, and after the layers were separated, the upper oil layer was taken out, the lower water layer was extracted with benzene, and the extract was combined with the above oil layer, dried, filtered, and distilled to obtain diethylaminochloroethane, Its yield is about 90%, and its boiling point is about 134°C.
[0031] After adding 20 mol of thiourea and 0.002 mol of tetramethylammonium bromide into 200 mol of water, 22 mol of diethyla...
Embodiment 2
[0033] Take 150 mol of thionyl chloride and add it to 200 mol of chloroform or carbon tetrachloride. Under external cooling conditions, take 100 mol of diethylaminoethanol and add it dropwise to the above solution at a temperature of 30 to 35° C. to form a reaction mixture. The reaction mixture was refluxed at 60°C for 4 hours, then the reaction mixture was cooled to room temperature, filtered, and vacuum-rotated to obtain powdered diethylaminochloroethane hydrochloride with a yield of 96% and a melting point of 210-212°C about.
[0034] After adding 50mol of thiourea and 0.005mol of benzyltripropylammonium chloride into 500mol of water to dissolve, add 60mol of diethylaminochlorethane hydrochloride in batches at a temperature of 35-40°C, and let the reaction The mixture is incubated and reacted at a temperature of 70-75° C. for 3 hours, and then the potassium hydroxide solution with a mass percentage concentration of 40% in the reaction mixture is adjusted to pH = 13, and the...
Embodiment 3
[0036] Take 200mol of benzene and 110mol of thionyl chloride to form a mixed solution, drop 100mol of diethylaminoethanol into the above mixed solution at a temperature of 35-40°C under external cooling conditions to form a reaction mixture, and then put the reaction mixture Reflux at 75°C for 2 hours, then cool the reaction mixture to 40°C, and add 400 mol of water dropwise. After the reaction mixture is cooled below 20°C, add dropwise sodium hydroxide with a concentration of 45% by mass to adjust the pH of the reaction mixture. value of 10, the reaction mixture was left standing, and after the layers were separated, the oil layer was taken out, and the lower water layer was extracted with benzene, the extract was combined with the above oil layer, dried, filtered, and distilled to obtain diethylaminochloroethane, which The yield is 90%, and the boiling point is about 134°C.
[0037] Get the benzyltrimethylammonium chloride of 30mol thiourea 0.001mol and be dissolved in the 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com