Full biodegradable amphipathic polysaccharides grafts as well as preparation method and use thereof
A kind of graft and amphiphilic technology, applied in the field of functional polymer materials, can solve the problems of unfavorable use of organic solvents, limited types, long reaction time and other problems of amphiphilic grafts, and achieve good biodegradability and biocompatibility Capacitance, mild reaction conditions, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
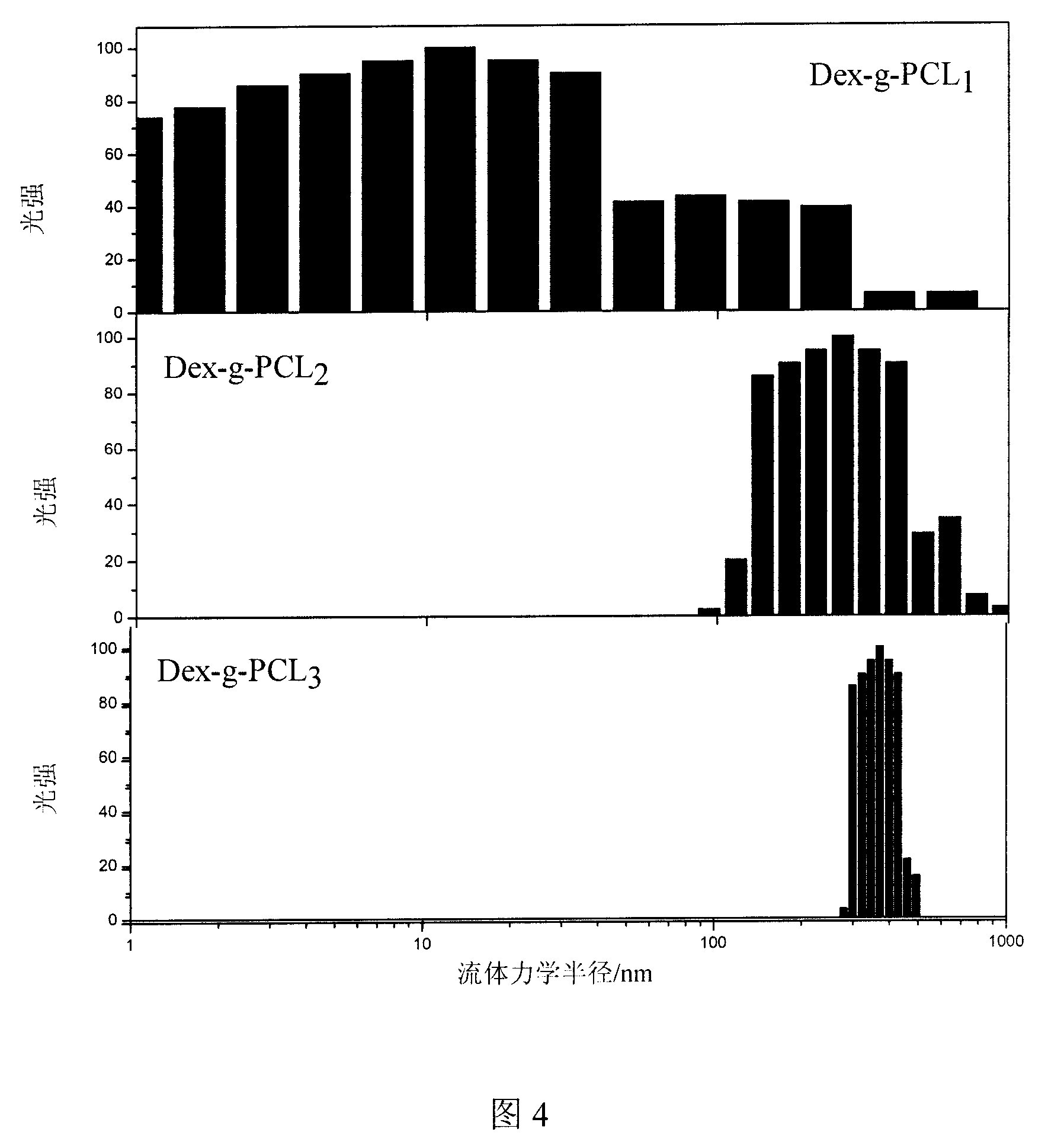
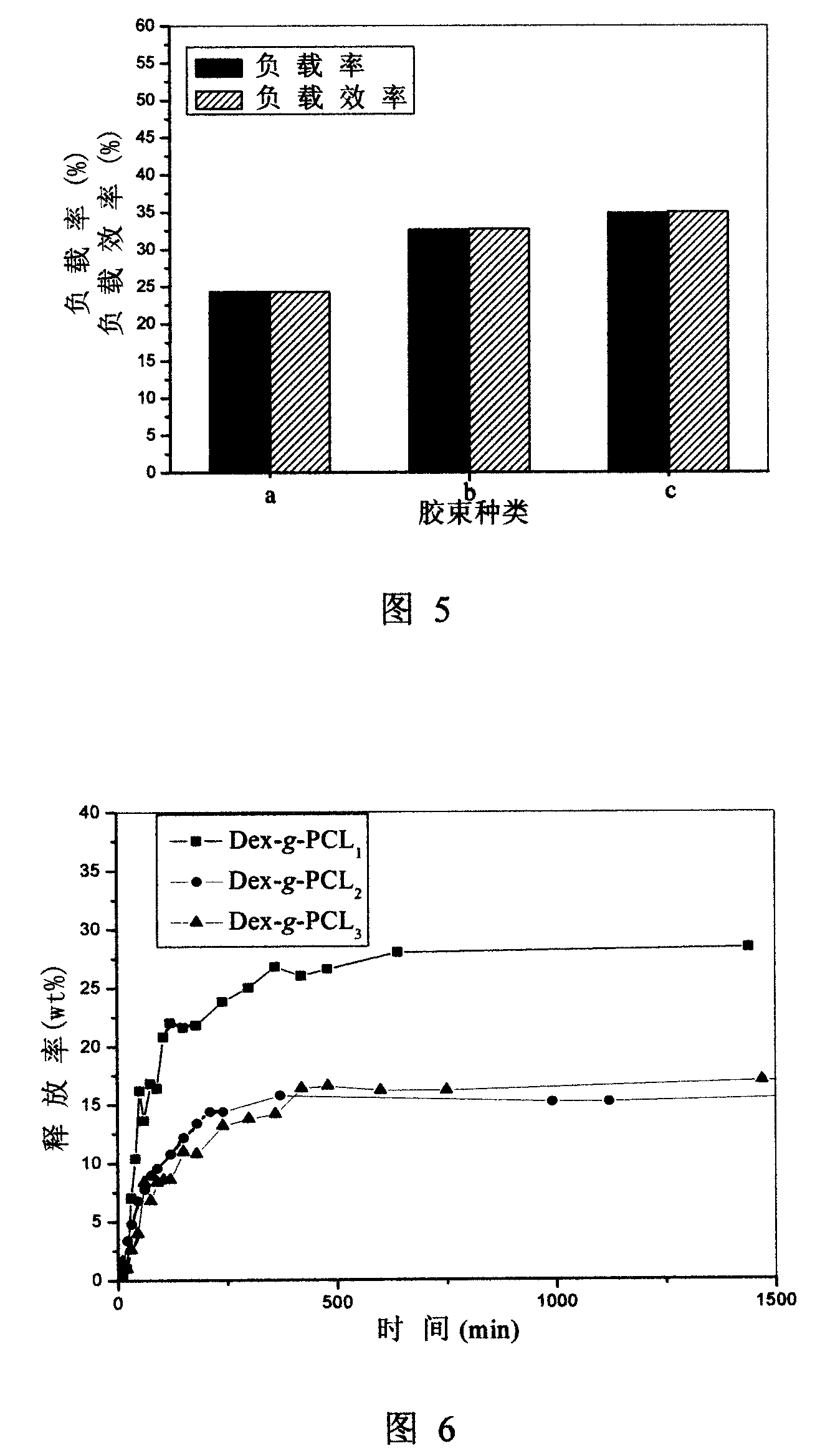
Image
Examples
Embodiment 1De
[0026] The preparation of embodiment 1Dex-g-PCL graft
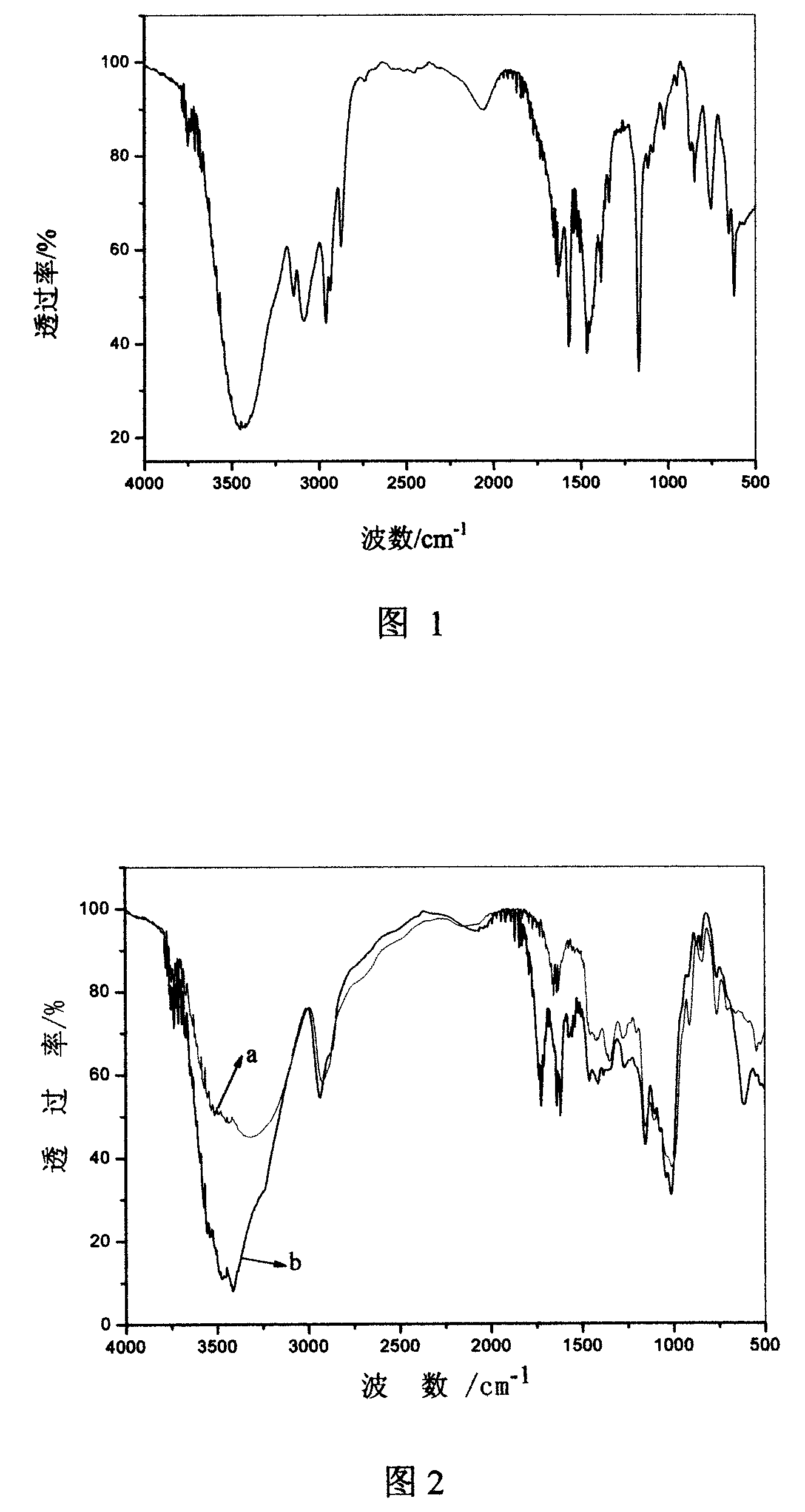
[0027](1) Synthesis of 1-methyl-3-butylimidazolium bromide ([BMIM]Br) ionic liquid: Weigh 16.42g (0.2mol) of N-methylimidazole and add it to a 250mL three-port In the flask, under electromagnetic stirring, 34.26 g (0.25 mol) of n-butane bromide which had been dried with phosphorus pentoxide and distilled was added dropwise, and stirred for 2 h after the dropwise addition was completed. Then use an oil bath to heat, control the temperature to 60°C, and react for 24 hours. After the reaction, cool to room temperature, and use a rotary evaporator at 90° C. for 2 h. Then, it was dried in a vacuum oven at 80° C. for 24 h to obtain 1-methyl-3-butylimidazolium bromide ([BMIM]Br) ionic liquid. spare. The yield was 90%. As shown in Figure 1 infrared spectrum: 3144cm -1 and 3087cm -1 It is the absorption peak of stretching vibration of C-H bond on the ring; 2967cm -1 、2927cm -1 and 2867cm -1 It is the characteristic absorp...
Embodiment 2D
[0029] The preparation of embodiment 2Dex-g-PCL graft
[0030] (1) Synthesis of 1-methyl-3-butylimidazolium bromide ([BMIM]Br) ionic liquid: Weigh 16.42g (0.2mol) of N-methylimidazole and add it to a 250mL three-port In the flask, under electromagnetic stirring, 27.4 g (0.2 mol) of n-butane bromide which had been dried with phosphorus pentoxide and distilled was added dropwise, and stirred for 3 h after the dropwise addition was completed. Then use an oil bath to heat, control the temperature to 55° C., and react for 22 hours. After the reaction, cool to room temperature, and use a rotary evaporator at 85° C. for 4 h. Then it was dried in a vacuum oven at 60° C. for 20 h to obtain 1-methyl-3-butylimidazolium bromide ([BMIM]Br) ionic liquid. spare. The yield was 86%. As shown in Figure 1 infrared spectrum: 3144cm -1 and 3087cm -1 It is the absorption peak of stretching vibration of C-H bond on the ring; 2967cm -1 、2927cm -1 and 2867cm -1 It is the characteristic absorp...
Embodiment 3D
[0032] The preparation of embodiment 3Dex-g-PCL graft
[0033] (1) Synthesis of 1-methyl-3-butylimidazolium bromide ([BMIM]Br) ionic liquid: Weigh 16.42g (0.2mol) of N-methylimidazole and add it to a 250mL three-port In the flask, under electromagnetic stirring, 41.11 g (0.3 mol) of n-butane bromide which had been dried with phosphorus pentoxide and distilled was added dropwise, and stirred for 1 h after the dropwise addition was completed. Then use an oil bath to heat, control the temperature to 65°C, and react for 20h. After the reaction, cool to room temperature, and use a rotary evaporator at 95° C. for 3 h. Then it was dried in a vacuum oven at 70° C. for 12 h to obtain 1-methyl-3-butylimidazolium bromide ([BMIM]Br) ionic liquid. spare. The yield was 93%. As shown in Figure 1 infrared spectrum: 3144cm -1 and 3087cm -1 It is the absorption peak of stretching vibration of C-H bond on the ring; 2967cm -1 、2927cm -1 and 2867cm -1 It is the characteristic absorption p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Effective particle size | aaaaa | aaaaa |
| Effective particle size | aaaaa | aaaaa |
| Effective particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com