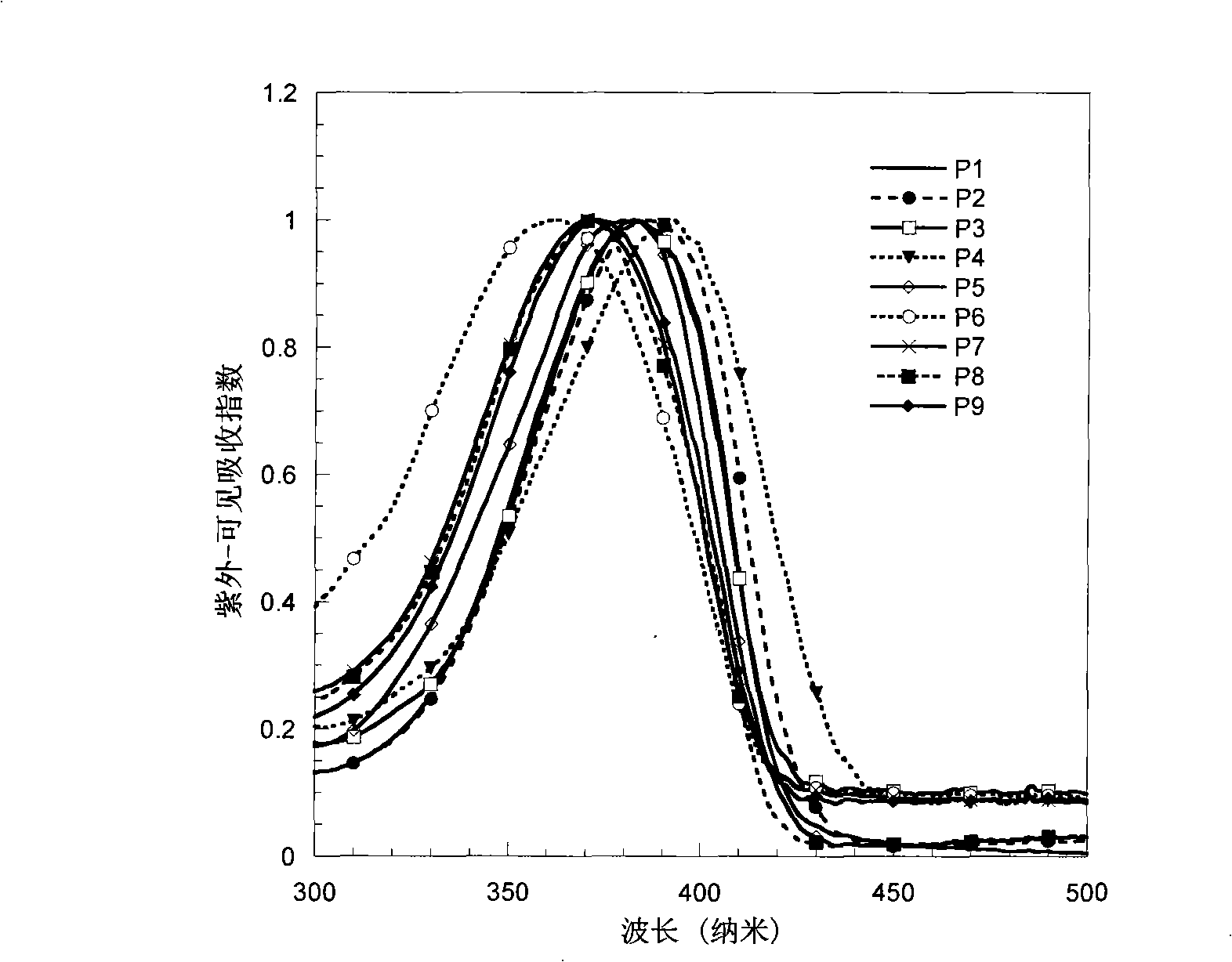
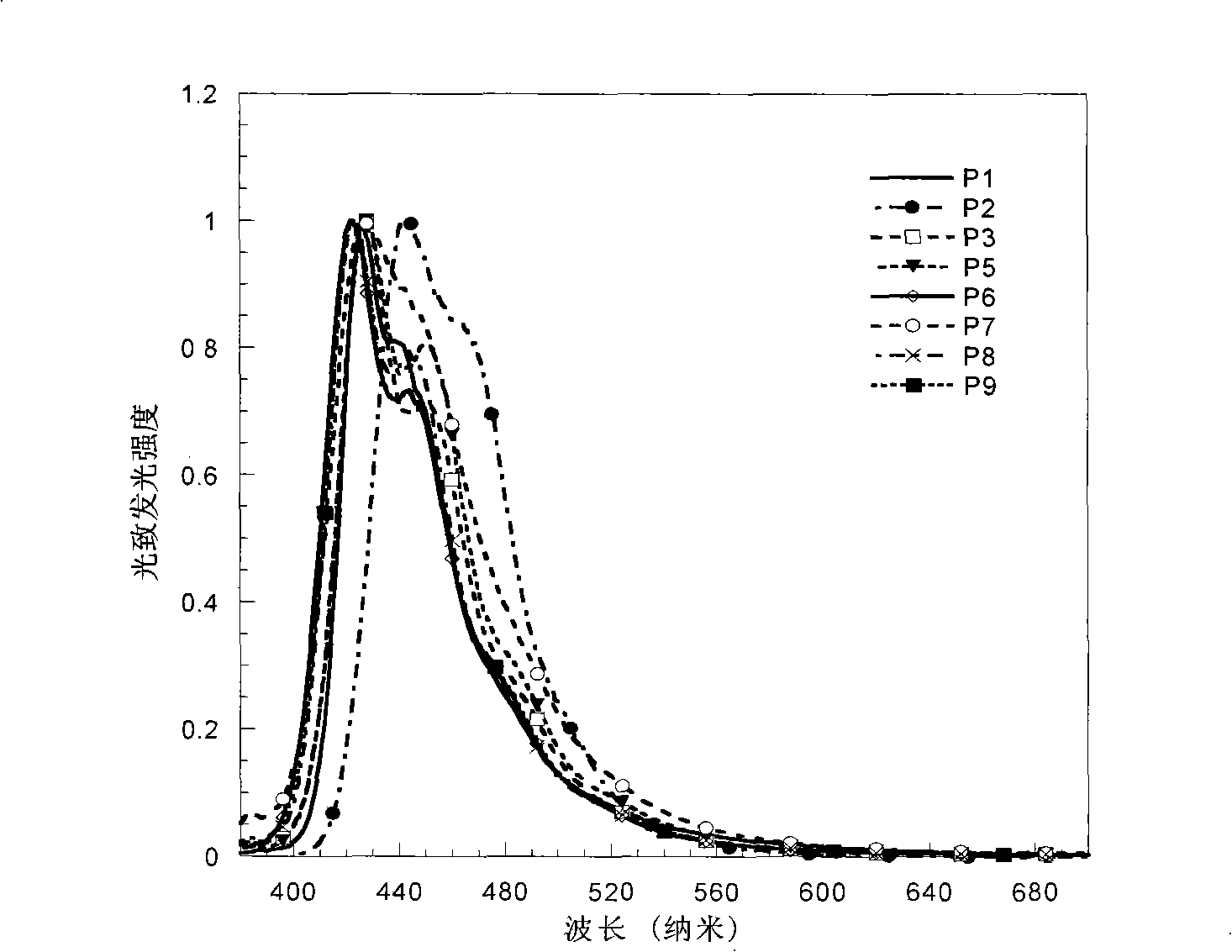
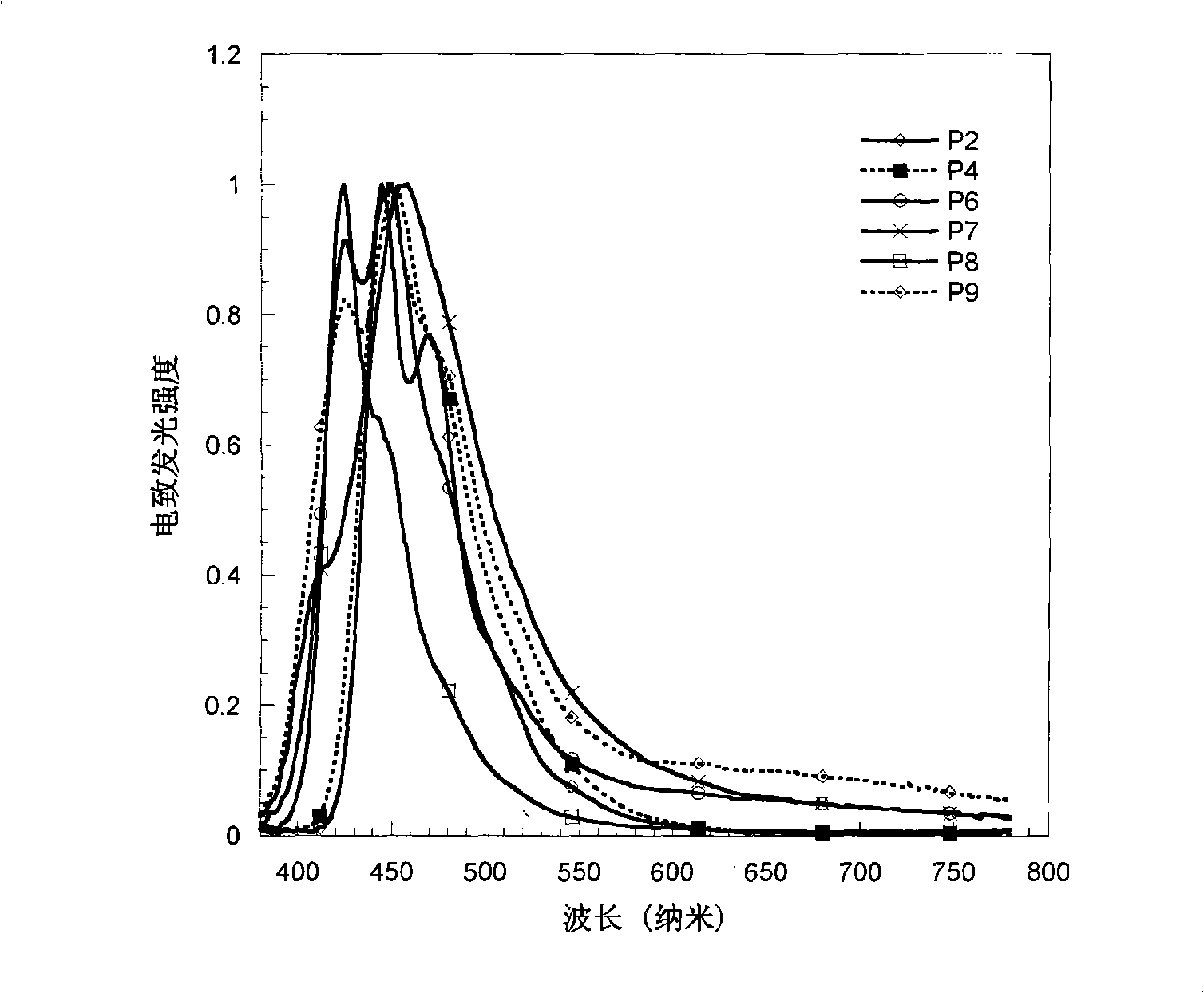
Electroluminescent spectrum-stable blue fluorene-based polymers as well as preparation method and uses thereof
An electroluminescence and polymer technology, applied in the field of blue fluorene polymers and their preparation, can solve the problems of luminescence spectral tailing, poor color purity and luminescence color stability, achieve good spectral stability and improve spectral stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1 2, the preparation of 7-dibromofluorene
[0060] Prepared according to the method of World Patent (WO 99 05184) and Chem.Mater.11(1997), 11083:
[0061] In a 250 ml three-necked flask, add 16.6 g (0.1 mol) of fluorene, 88 mg (1.57 mmol) of iron powder, and 100 ml of chloroform. After cooling in an ice-water bath, 35 ml of a bromine 35.2 g (0.22 mol) / chloroform mixed solution was added dropwise. The temperature in the bottle does not exceed 5°C during the dropwise addition. After the reaction was completed, it was filtered and recrystallized from chloroform to obtain 26.9 g of white crystals with a yield of 83%. 13 C NMR and GC-MASS tests showed that it was the target product.
[0062]
Embodiment 2
[0063] Example 2 Preparation of 2,7-dibromo-9,9-dialkylfluorene
[0064] Take the preparation of 2,7-dibromo-9,9-di-n-octylfluorene as an example to illustrate
[0065] Add 9.7 grams (0.03 moles) of 2,7-dibromofluorene, 0.07 grams (0.3 mmoles) of benzyltriethylammonium chloride, 90 milliliters of dimethyl sulfoxide, and 45 milliliters of aqueous sodium hydroxide ( 50%). Stir vigorously at room temperature to form a suspension. 12.5 g (65 mmol) of 1-bromo-n-octane was slowly added dropwise, and stirring was continued for 3 hours, followed by extraction with diethyl ether. The ether phase was washed with saturated aqueous sodium chloride and dried over anhydrous magnesium sulfate. The solvent was evaporated, and the product was purified by column chromatography using petroleum ether as the eluent to obtain white crystals. 13 C NMR and GC-MASS tests showed that it was the target product.
[0066]
Embodiment 3
[0067] Example 3 Preparation of 2,7-diboronate-9,9-dialkylfluorene
[0068] According to the method disclosed in Macromolecules 30 (1997) 7686, the preparation of 2,7-diboronate-9,9-di-n-octylfluorene is taken as an example to illustrate.
[0069] 5.6 g (10.22 mmol) of 2,7-dibromo-9,9-di-n-octylfluorene and 130 ml of anhydrous tetrahydrofuran were added to a 500 ml three-necked flask. Under argon protection, 20 ml (32 mmol) of n-butyllithium / n-hexane solution (1.6M) was added dropwise at -78°C, and stirred at -78°C for 2 hours. Then 25 ml (123 mmol) of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-ethylenedioxy borate was added rapidly, and stirring was continued at -78°C 2 hours. The reaction mixture was gradually warmed to room temperature, and the reaction was stirred for 36 hours. The reaction mixture was poured into ether / water, extracted with ether, washed with aqueous NaCl, and dried over anhydrous magnesium sulfate. The solvent was evaporated, and the residue was recrysta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com