Oral instant quick-effective emulsion membrane and three-dimensional printing preparation method
An oral instant-dissolving and drug-film technology, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and sheet-like transportation, to achieve a high degree of automation and a flexible preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
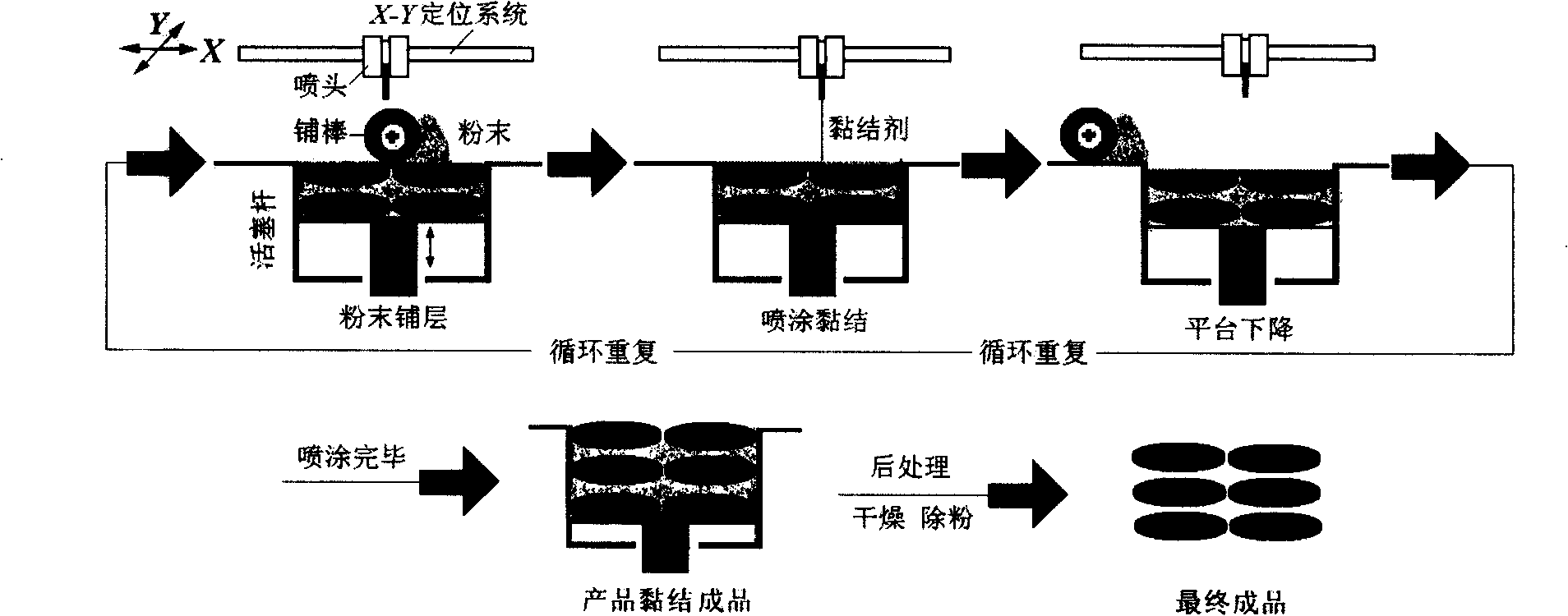
Image
Examples
Embodiment 1
[0028] Example 1: 3D printing powder formulation
[0029]Sieve lactose, polyvinylpyrrolidone, microcrystalline cellulose, and mannitol powder one by one, except for microcrystalline cellulose (12-20 μm), select powders with a particle size distribution between 75-100 μm and mix them evenly in proportion , to make a layered mixed powder, and the composition content is as follows by weight percentage:
[0030] Lactose 20 parts
[0031] Polyvinylpyrrolidone K30 20 parts
[0032] Mannitol 20 parts
[0033] Microcrystalline cellulose 40 parts
Embodiment 2
[0034] Example 2: Preparation of printing solution and selection of some process parameters
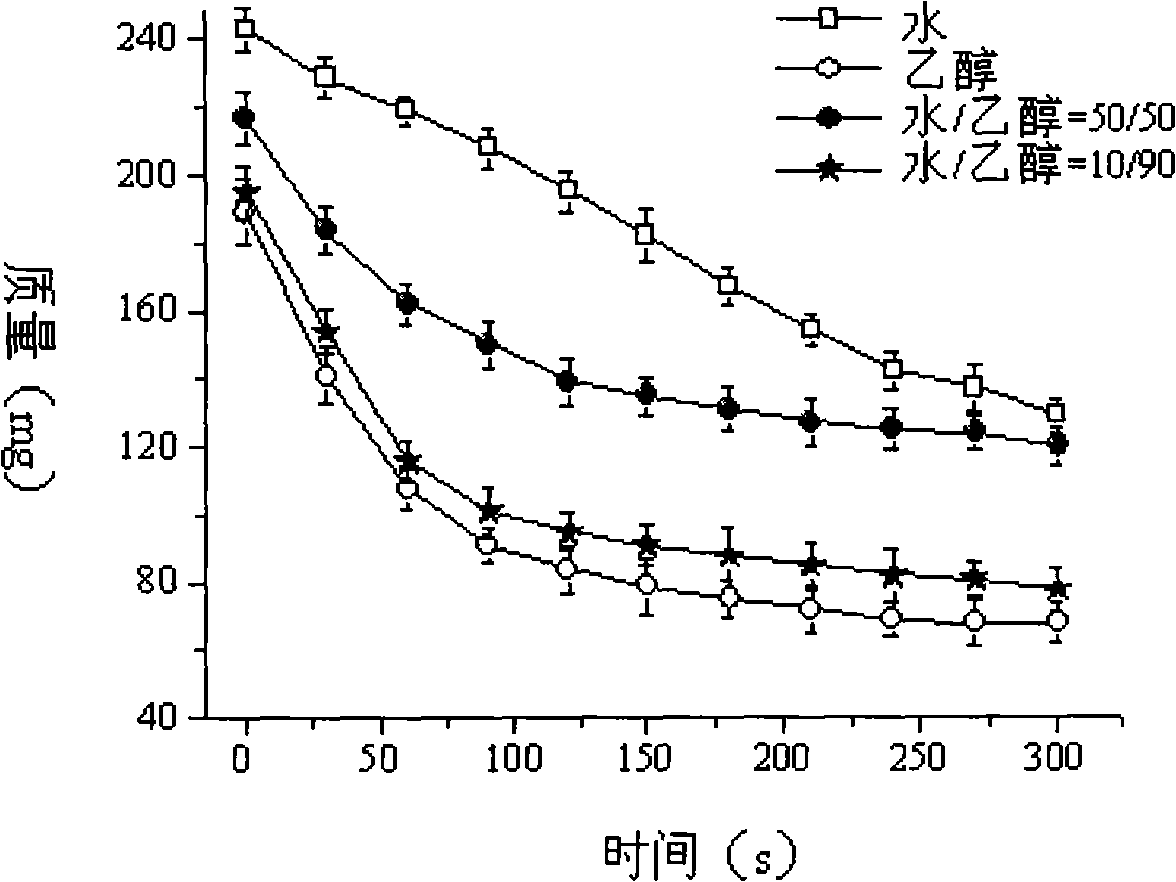
[0035] Since the polyvinylpyrrolidone K30 in the powder is easily soluble in ethanol. Mannitol and lactose are soluble in water, so the mixture of the two is selected as the solvent of the printing solution. Put a 12cm watch glass on the 1 / 10,000th electronic balance, put 2.0g of mixed powder into it, perform layering, and adjust to zero. At room temperature, use a quantitative spray bottle to spray the tested printing solution twice continuously, record the initial mass of the sprayed printing solution, and record the weight every 30s, repeat 6 times, take the average value, and compare the quality change with time drawing.
[0036] Such as image 3 As shown, water evaporates from the mixed powder at a relatively constant rate of 0.2289 mg s -1 , while ethanol and water-ethanol mixture have a linear fast volatilization stage at the beginning, and then the volatilization rate beco...
Embodiment 3
[0041] Embodiment 3: prepare quick-dissolving quick-acting drug film
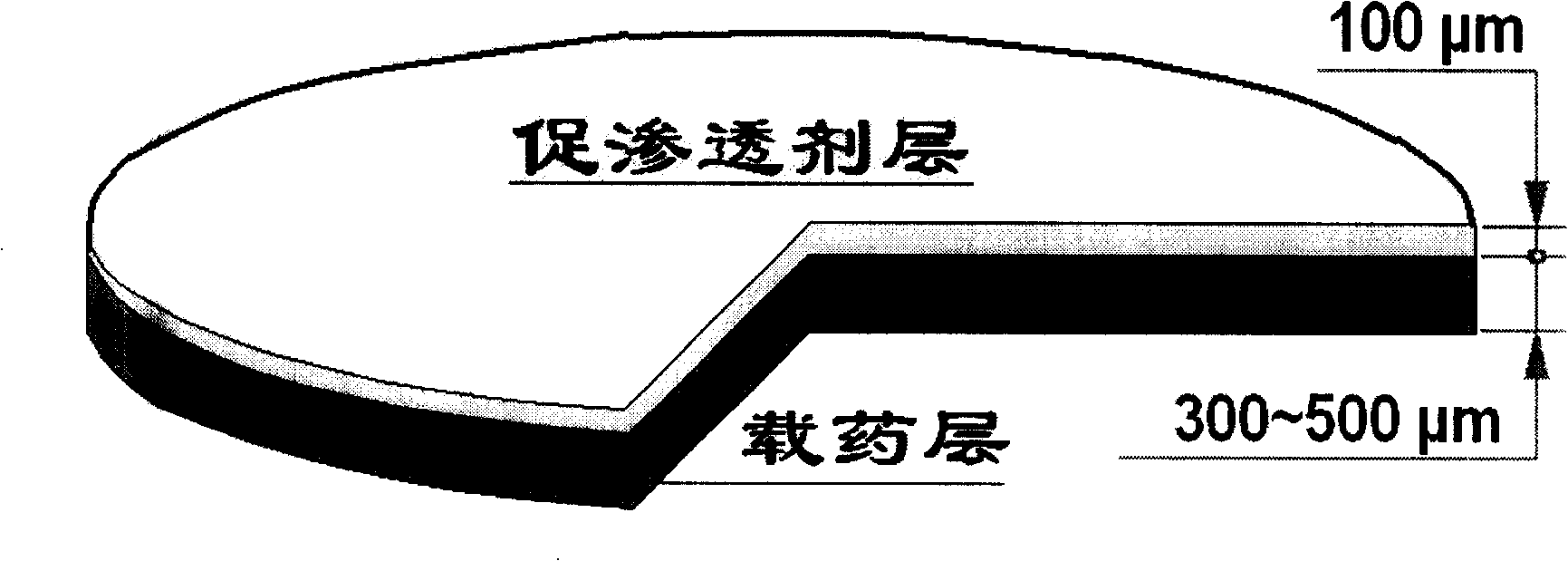
[0042] The operation and preparation are directly controlled by the computer terminal output instructions. Spread a layer of mixed powder with a thickness of 100 μm, spray 2 times of 90% ethanol aqueous solution containing 5% sodium lauryl sulfate as a penetration enhancer, and form the bottom surface of the drug film, and then the piston rod drives the powder bed of the workbench to descend as a whole, Prepare a new layer of powder.
[0043] The mixed powder of the subsequent 3 to 5 layers of laying remains unchanged, and the thickness of the laying layer is 100 μm. The powder is bonded and formed in a selective area with 90% ethanol aqueous solution containing 5% of the drug ibuprofen, and each layer is sprayed twice to form a carrier. Drug area, the diameter of the drug film is 18mm. Finally, the obtained drug film is dried and powder-removed to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com