Novel preparation of budesonide
An organic solvent and recrystallization technology, applied in steroids, respiratory diseases, organic chemistry, etc., can solve the problems of difficult strain source and screening, cumbersome steps, and high cost of industrial production, so as to facilitate the promotion of industrial production, High bioavailability and the effect of improving product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
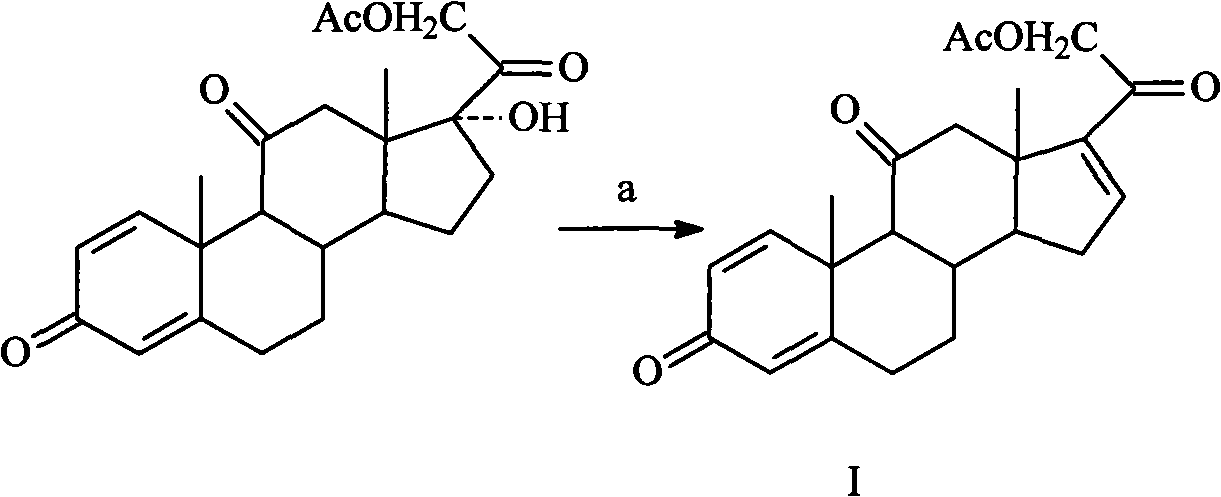
[0025] Embodiment 1: the preparation of intermediate I
[0026] Add 100g of prednisone acetate (0.25mol), 1000ml of DMF, 200ml of acetic anhydride, and 10g of 5-sulfosalicylic acid into the reaction flask slowly in turn, reflux for 8 hours, cool to below 5°C, pour into 15 liters of ice water, and place overnight , use NaOH to adjust the pH value to 6.0-7.0, then add 50g potassium acetate, add it to the reaction flask, react at 100-105°C for 6-7 hours under nitrogen protection, cool, pour into 8L ice water, place overnight, and suction filter , washed with water, and dried to obtain 92 g of crude product, which was recrystallized with chloroform to obtain 76 g of off-white crystals, yield 79.6%, mp 207-209°C.
Embodiment 2
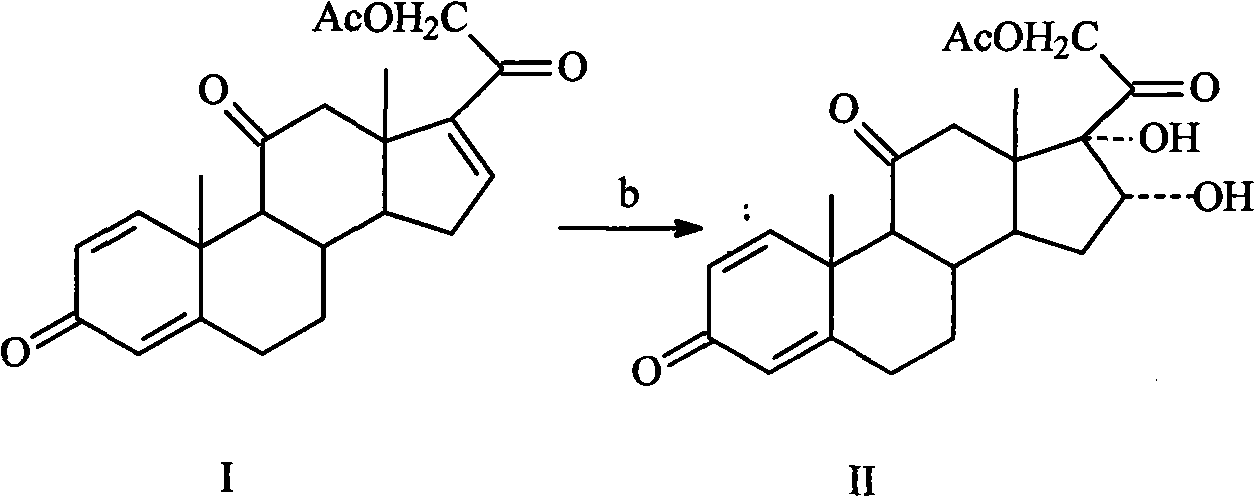
[0027] Embodiment 2: the preparation of intermediate II
[0028] Add 50g of intermediate I (0.131mol), 3000ml of acetone into the reaction flask, dissolve, cool the mixture to below 0°C, add 80% H 2 o 2 100ml, controlled the reaction temperature at -10°C and stirred for 10 hours, then concentrated under reduced pressure to obtain 44g of oil, with a yield of 80.8%.
Embodiment 3
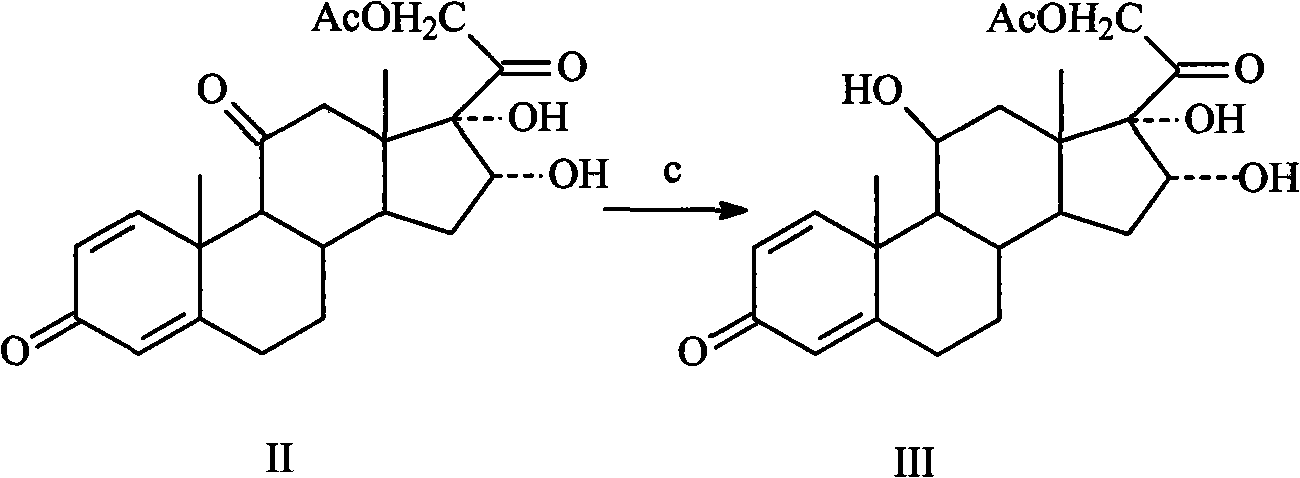
[0029] Embodiment 3: the preparation of intermediate III
[0030] Add 100g of intermediate II to 500ml of chloroform solution, add 200ml of tetrahydrofuran, slowly add 12g of sodium borohydride under stirring, stir, and control the reaction temperature at -5°C for 4 hours. After the reaction is complete, pour it into saturated saline, Stand overnight, filter, wash with water, and dry to obtain 83g of crude product. Recrystallize with 400ml of methanol-n-hexane (1:2) solution to obtain 72g of off-white crystals, yield 71.7%, mp195-201°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com