Method for analyzing ferulaic acid butyric glyceride by high performance liquid chromatography
A technology of glyceryl butyrate and high-performance liquid chromatography, which is applied in the field of chromatographic separation, can solve the problems of researchers, difficulties in controlling the reaction process, isomers, etc., reduce the number of experiments, and the method is simple, fast and simplified Effect of separation and purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
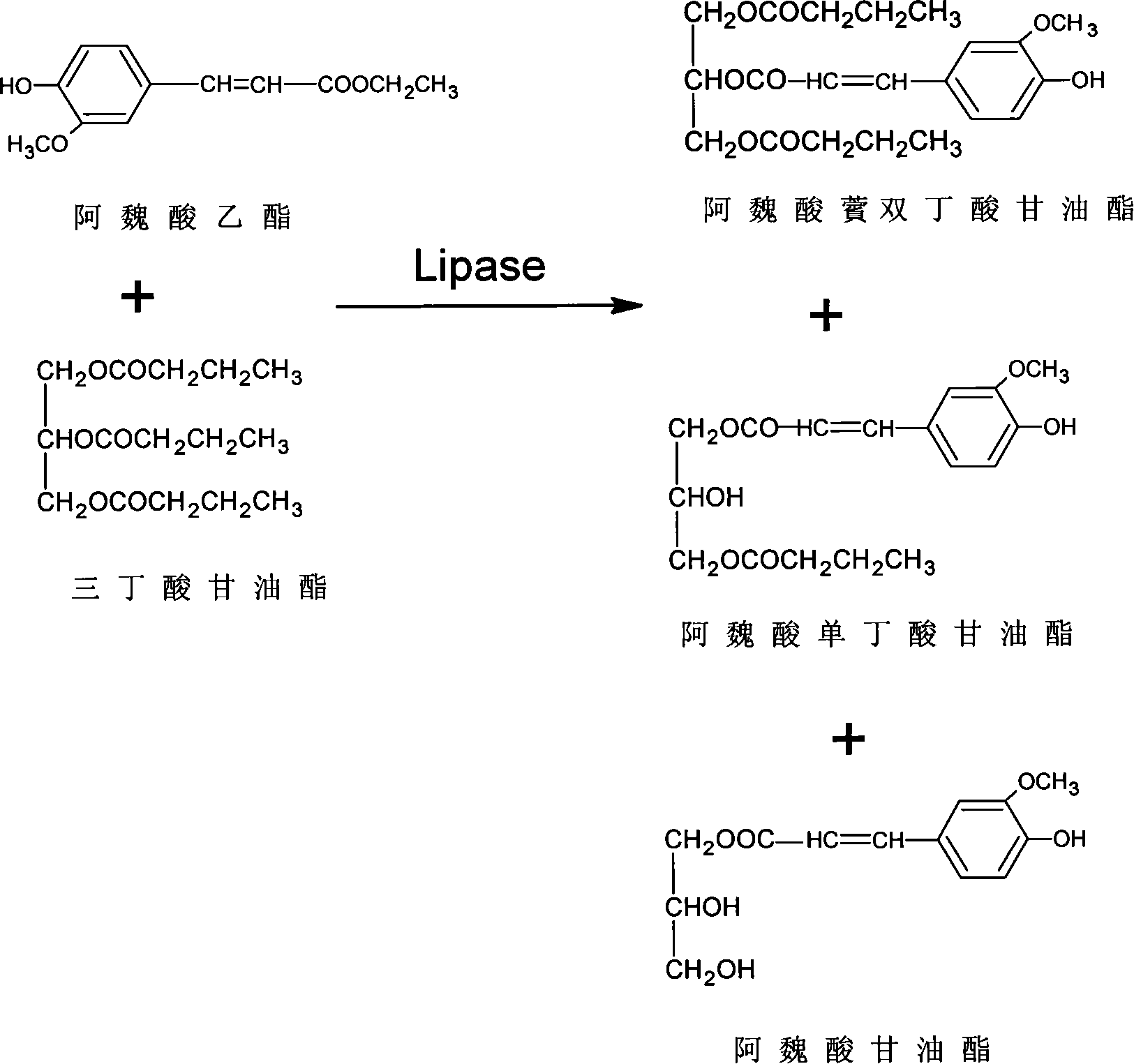
[0028] Esters, 120mg Novozym435 lipase, 5mL tert-butanol, shake in an air bath at 50°C at 150r / min for 120h, and remove the enzyme by filtration.
[0029] 2. Pretreatment for the analysis of ferulic acid butyrin by high performance liquid chromatography
[0030] The reaction medium isobutanol was removed by rotary evaporation of the above reaction mixture, and unreacted tributyrin was extracted with anhydrous ether / n-hexane (80 / 20, V / V). Finally, the residual organic medium in the reaction system was removed by nitrogen purging. Dissolve 1 mg of the mixed product in 1 mL of methanol, filter through a microporous membrane with a pore size of 0.45 μm, and set aside.
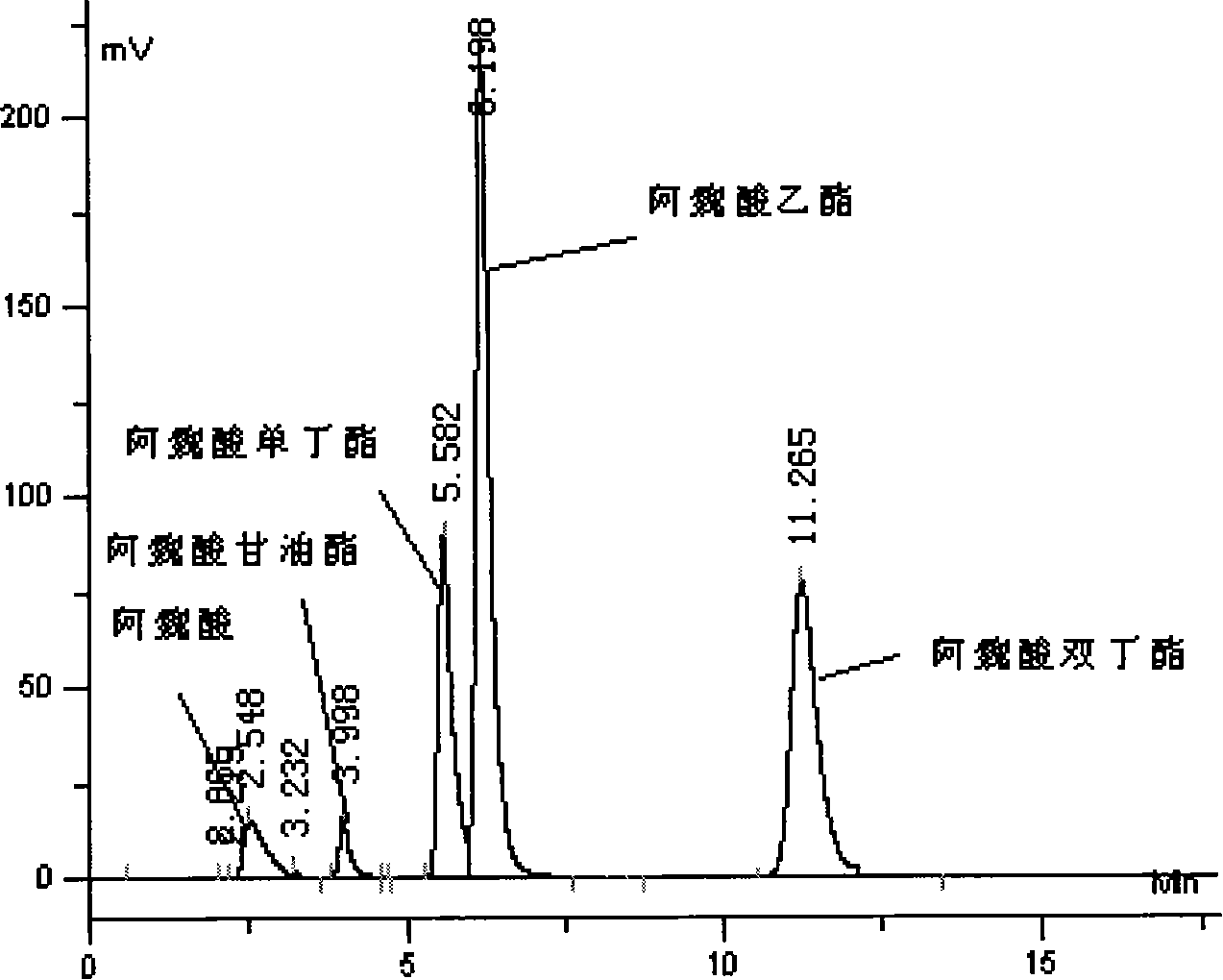
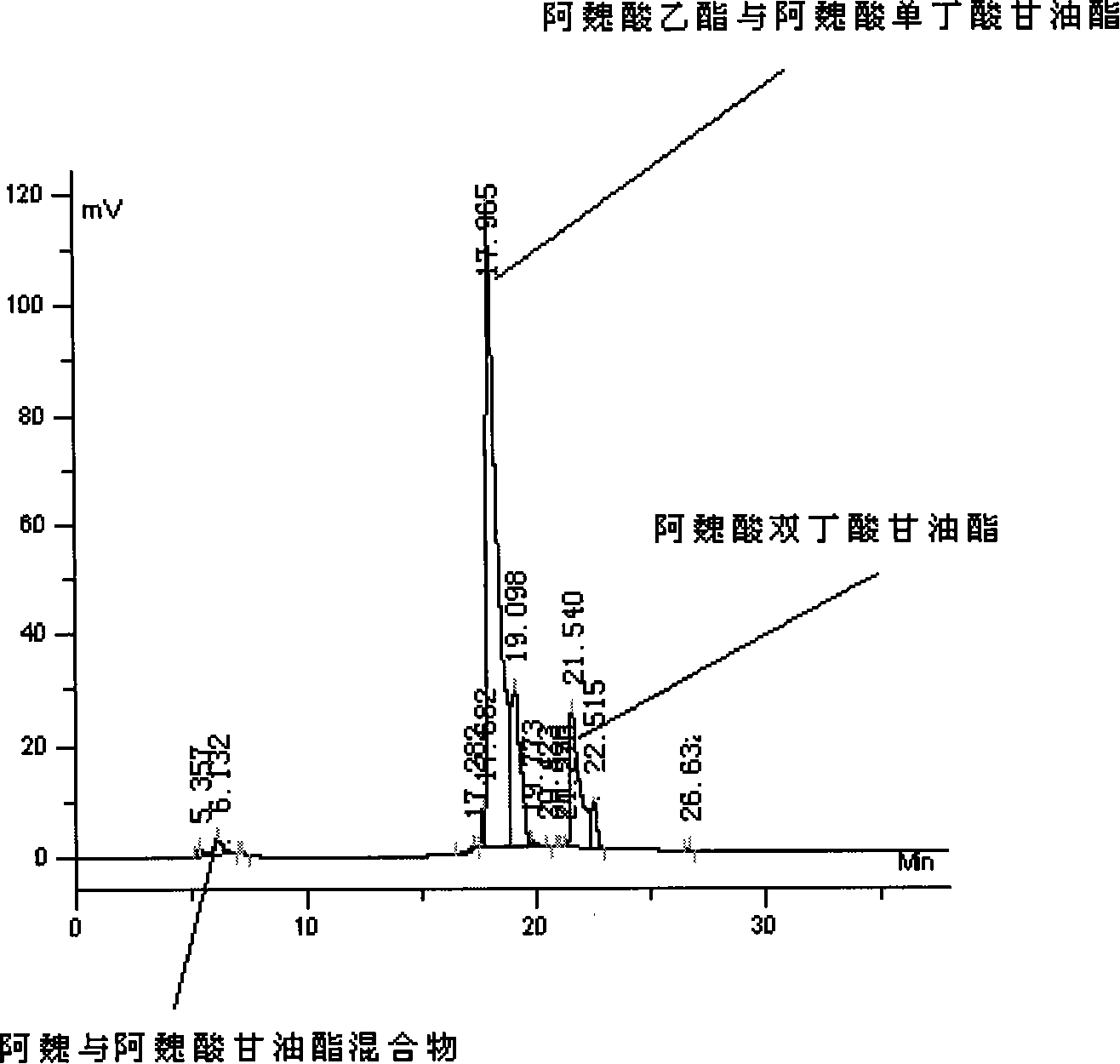
[0031] 3. Separation and analysis of ferulic acid butyric acid mixture by high performance liquid chromatography
[0032] (1) Take 10 μL of the reaction sample, dilute it to 1 mL with HPLC grade methanol, and then take 5 μL of the sample through a microsampler and add it to the injection valve.
[0033] (2) Mobi...
Embodiment 2
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com