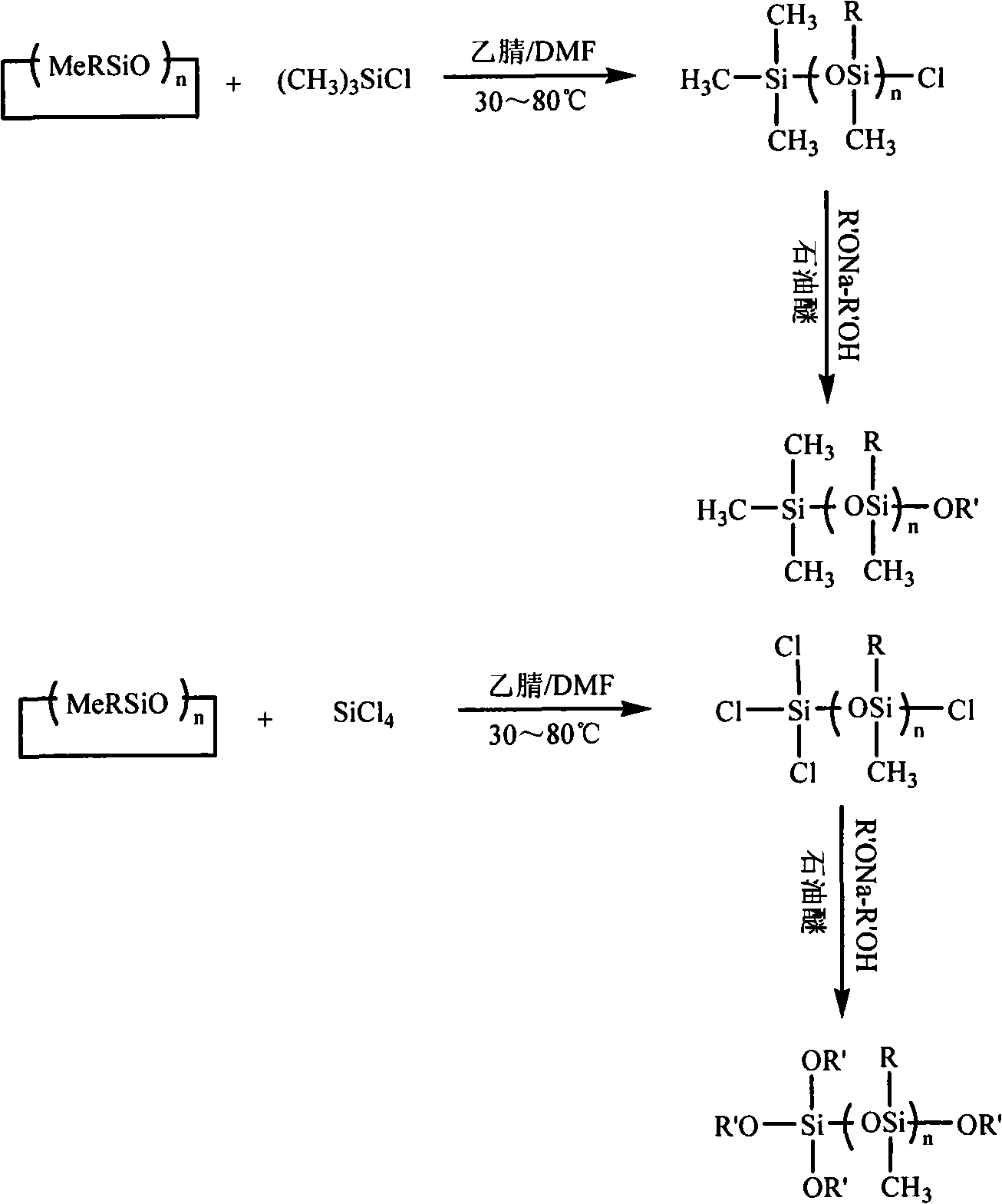
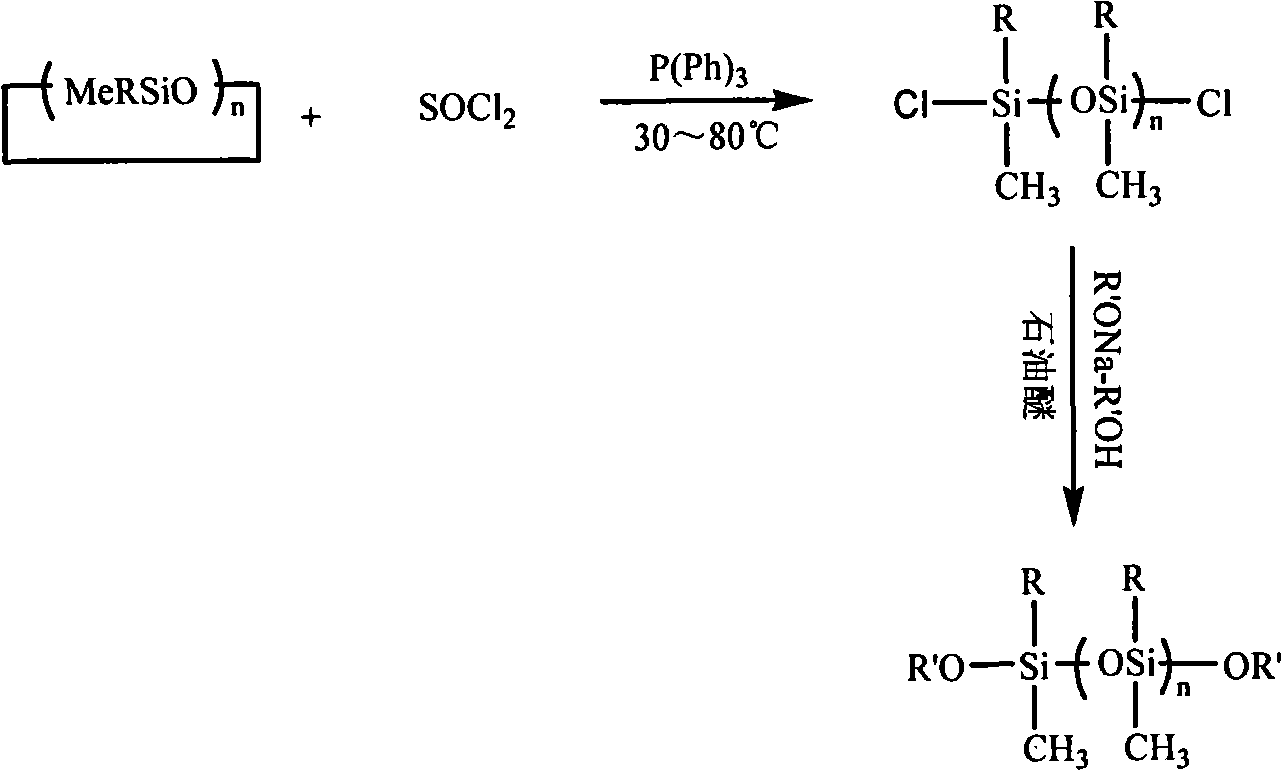
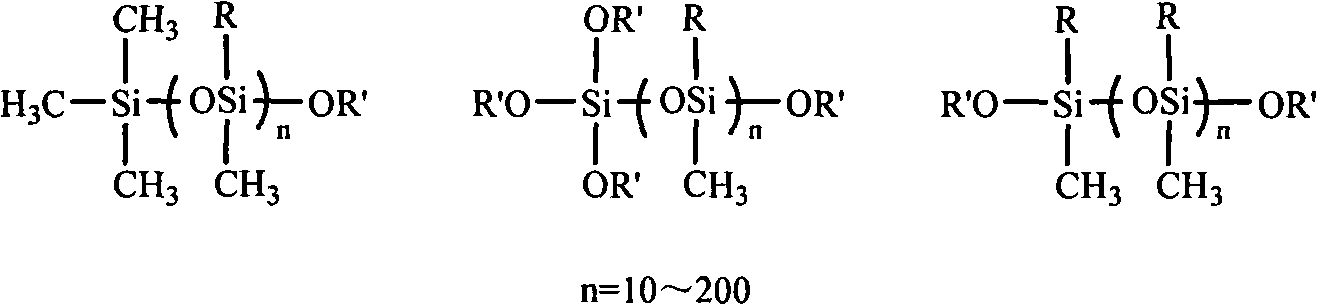Alkoxy end-capped linear polysiloxane resin acceptor and synthetic method thereof
A polysiloxane coupling agent and alkoxy-terminated technology, which is applied in the field of alkoxy-terminated linear polysiloxane coupling agent and its preparation, can solve the problems of high price, high preparation cost, high reactivity, etc. problem, to achieve good durability, overcome poor heat resistance, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In a 250ml three-neck flask equipped with a constant pressure dropping funnel, drying tower, magnetic stirring and heating reflux device, transfer 0.09ml N,N-dimethylformamide, 4.5ml acetonitrile, 6.4ml tetrachloride Si, stir vigorously to mix well. Transfer 77.4ml of octamethylcyclotetrasiloxane to the constant pressure dropping funnel, start adding octamethylcyclotetrasiloxane dropwise when the temperature of the system rises to 30°C, and after constant temperature reaction for 16 hours, distill off the solvent and catalyst under reduced pressure, A monochloro-terminated linear polymethylsiloxane was obtained. The resulting product is a mixture whose structural formula Me 3 Si(OSiMe 2 )n Cl.
[0038] After the above synthesis is completed, use petroleum ether (boiling range 30-60°C) as a solvent, and slowly add excess sodium methoxide-methanol saturated solution and monochlorine-terminated linear polysiloxane dropwise at the same time. After reflux reaction for 5 h...
Embodiment 2
[0041] In a 250ml three-neck flask equipped with a constant pressure dropping funnel, drying tower, magnetic stirring and heating reflux device, transfer 0.71ml N,N-dimethylformamide, 16ml acetonitrile, 14.5ml silicon tetrachloride with a syringe needle , stir vigorously to mix well. Transfer 77.4ml of octamethylcyclotetrasiloxane to the constant pressure dropping funnel, start adding octamethylcyclotetrasiloxane dropwise when the temperature of the system rises to 60°C, and react at constant temperature for 12 hours, distill off the solvent and catalyst under reduced pressure, The α-monochloro-ω-trichloro linear polymethylsiloxane was prepared. The resulting product is a mixture, which is a colorless transparent liquid with a simplified structure of Cl 3 Si(OSiMe 2 ) n Cl.
[0042] After the above synthesis is completed, using petroleum ether (boiling range 30-60°C) as a solvent, slowly add excess sodium ethoxide-ethanol saturated solution and α-monochloro-ω-trichloro lin...
Embodiment 3
[0044] In a 250ml three-necked flask equipped with a constant pressure dropping funnel, a drying tower, a magnetic stirring device, and a heating reflux device, transfer 1.7ml N, N-dimethylformamide, 22.5ml acetonitrile, and 64ml trimethylchlorosilane with a syringe needle. , stir vigorously to mix well. Transfer 64ml of octamethylcyclotetrasiloxane to the constant pressure dropping funnel, start adding octamethylcyclotetrasiloxane dropwise when the temperature of the system reaches 80°C, and react at constant temperature for 8 hours, distill off the solvent and catalyst under reduced pressure to prepare Obtain monochlorine-terminated linear polymethylsiloxane. The resulting product is a mixture, which is a colorless transparent liquid, and its structural formula Me 3 Si(OSiMe 2 ) n Cl.
[0045] After the above synthesis is completed, use petroleum ether (boiling range: 30-60°C) as solvent, and slowly add excess sodium methoxide-methanol saturated solution and monochlorine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com