Preparation method of manganese-zinc ferrite magnetic nano microsphere
A technology of manganese zinc ferrite and magnetic nanometers, which is applied in the field of preparation of magnetic nanospheres, can solve the problems of many process steps, uncontrollable shape, impure crystal phase, etc., and achieve simple preparation process and simple production equipment requirements , crystal phase pure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]Weigh 1.351g ferric chloride, 0.1487g zinc nitrate hexahydrate, 0.338g manganese sulfate monohydrate, add to a three-necked flask, then add 50ml ethylene glycol, 3.6g anhydrous sodium acetate, 1ml polyethylene glycol, 0.01g The polyvinylpyrrolidone was mechanically stirred for 30 minutes at a rotation speed of 350 rpm. After it was completely dissolved, the above solution was poured into a reaction kettle, and the temperature was raised to 200°C for 12 hours of reaction. After the reaction was completed, the product was collected with a magnet, washed with deionized water, and then dried at 60° C. for 15 hours to obtain magnetic nanospheres.
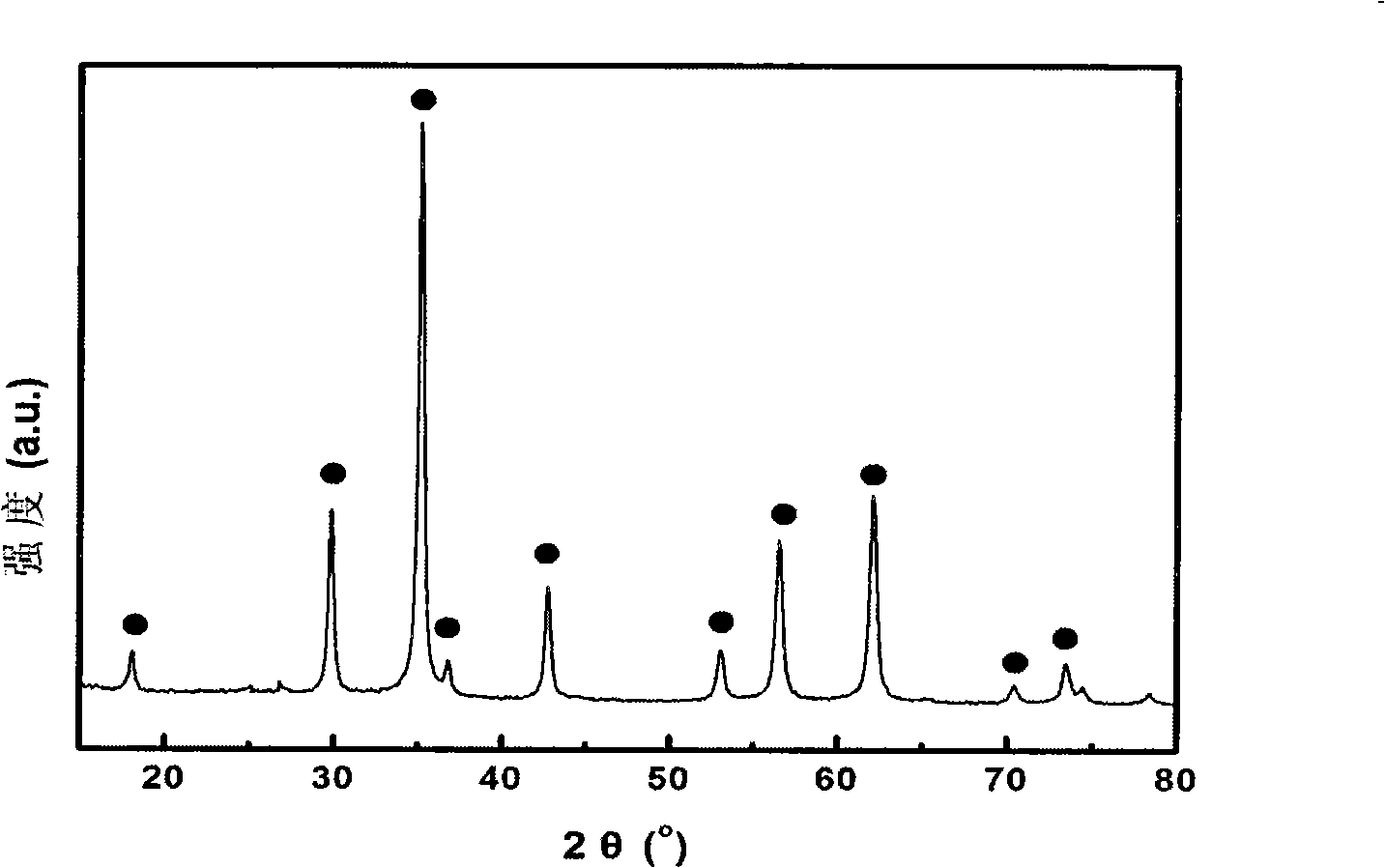
[0029] figure 1 For the X-ray diffraction pattern of the magnetic nano-microspheres synthesized in this embodiment, it can be seen that the diffraction peaks are consistent with the diffraction peaks of the manganese-zinc ferrite in the cubic phase, and no diffraction of other oxides belonging to manganese, zinc, and iron is found....
Embodiment 2
[0031] Weigh 1.351g of ferric chloride, 0.2975g of zinc nitrate hexahydrate, 0.2535g of manganese sulfate monohydrate, add to a three-necked flask, then add 55ml of ethylene glycol, 3.8g of anhydrous sodium acetate, 1.1ml of polyethylene glycol, 0.012 g of polyvinylpyrrolidone, mechanically stirred at 450 rpm for 20 minutes, and after being completely dissolved, pour the above solution into a reaction kettle, raise the temperature to 210° C., and react for 11 hours. After the reaction was completed, the product was collected with a magnet, washed with deionized water, and then dried at 50° C. for 24 hours to obtain magnetic nanospheres.
[0032] The X-ray test results show that the diffraction peaks are consistent with the diffraction peaks of the cubic phase manganese zinc ferrite, and no diffraction peaks belonging to other oxides of manganese, zinc, and iron are found, indicating that there is no impurity phase. Analysis and comparison by XRD data analysis software (JADE5.0...
Embodiment 3
[0034] Weigh 1.351g of ferric chloride, 0.4462g of zinc nitrate hexahydrate, 0.169g of manganese sulfate monohydrate, add to a three-necked flask, then add 45ml of ethylene glycol, 4 grams of anhydrous sodium acetate, 0.8ml of polyethylene glycol, 0.01 g of polyvinylpyrrolidone, mechanically stirred for 20 minutes at a rotational speed of 450 rpm, and after being completely dissolved, pour the above solution into a reaction kettle, raise the temperature to 190° C., and react for 10 hours. After the reaction was completed, the product was collected with a magnet, washed with deionized water, and then dried at 55° C. for 20 h to obtain magnetic nanospheres.
[0035] The X-ray test results show that the diffraction peaks are consistent with the diffraction peaks of the cubic phase manganese zinc ferrite, and no diffraction peaks belonging to other oxides of manganese, zinc, and iron are found, indicating that there is no impurity phase. The comparison was analyzed by XRD data ana...
PUM
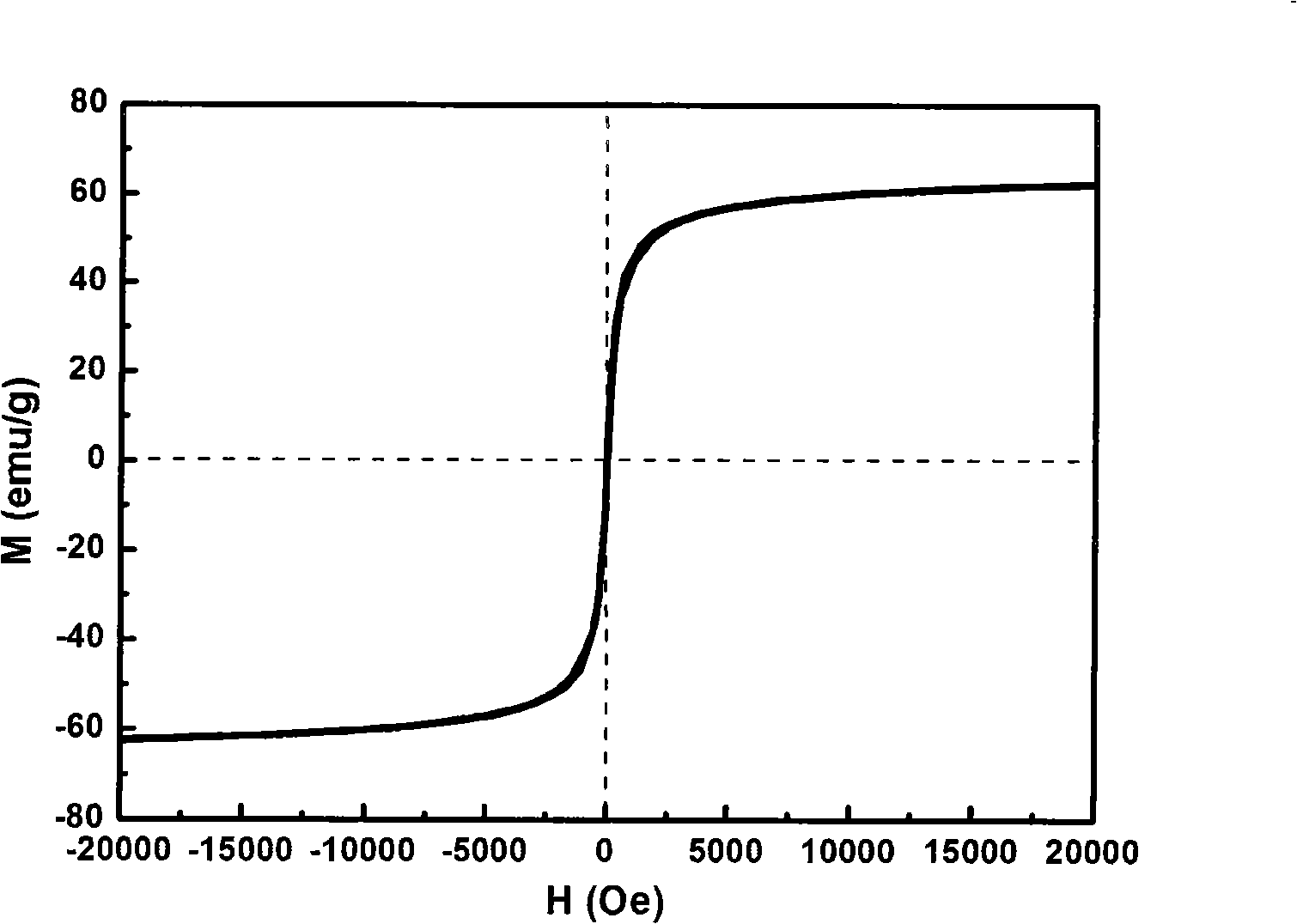
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com